Proposed Solutions to Further Improve the Regulatory Landscape for ATMPs in Europe
Cell Gene Therapy Insights 2018; 4(6), 535-544.
10.18609/cgti.2018.054
In support of the combined efforts by the European Commission (EC), the European Medicines Agency (EMA) and national competent authorities (NCA) to adapt and improve the regulatory framework to the development and patient access to Advanced Therapy Medicinal Products (ATMPs), the Alliance for Regenerative Medicine (ARM) has worked with its members to identify some of the main regulatory hurdles and potential solutions that would facilitate the development of ATMPs in Europe. A series of position papers have been published in 2017-2018 to outline ARM recommendations. Hospital exemption and GMO requirements for clinical trials with gene therapies have been identified as major obstacles for ATMP developers due to divergent interpretations and implementations by NCA. Other potential improvements in the regulatory framework include a convergence between the different Member States of donor testing and procurement requirements for cells and tissues used as starting materials for the manufacturing of ATMPs, a pragmatic approach with licensing requirements and regulatory processes, and a greater engagement and coordination in Europe for the development of international standards. ARM calls for pan-European convergence of requirements and, for each of the major issues identified, has defined recommendations for implementation by the EC, EMA or NCA to ensure faster patients access to these transformative medicines without compromising safety and efficacy aspects.
Submitted: Jun 5th 2018 Published: 7th July 2018
On 27 May 2016, the European Medicines Agency (EMA) convened a multi-stakeholder meeting to explore ways to foster Advanced Therapies Medicinal Products (ATMPs) development and expand patient access. The meeting was attended by leading academics and researchers, incubators and consortium organizations, representatives from patients and healthcare professionals’ organizations, small and large pharmaceutical companies, the investment community, health technology assessment (HTA) bodies, national competent authorities (NCAs) and the European Commission (EC). The Alliance for Regenerative Medicine (ARM), a multi-stakeholders organization comprising industrial and non-industrial developers, service providers, patients’ organizations and others, is a leading advocate for advanced therapy products that meet the needs of patients worldwide by bringing them innovative, safe, effective and high-quality products. ARM participated in this meeting as well as in the survey sent prior to it. Further to this meeting, the EMA published a report summarizing the main ideas and solutions proposed during the meeting as well as responses sent ahead of the meeting via a questionnaire [1]. This report has been used to enrich on-going initiatives by the EMA and the Committee for Advanced Therapies (CAT) and prioritize actions that could be undertaken to improve the regulatory framework in the European Union. A list of the issues raised, and on-going or planned activities based on the output of the stakeholder consultation was released in February 2017 [2]. Following this, the European Commission-DG Health and Food Safety together with the EMA published an action plan on ATMPs listing proposed actions to improve the regulatory framework for ATMPs in collaboration with the authorities of the Member States [3].
In parallel to the exercise undertaken by the European Commission (EC) and the EMA, ARM has continued to collect feedback from its members to identify potential solutions to the major issues associated with the development and patient access to ATMPs in Europe, based on their concrete experience. As a result, a series of position papers was published dealing with specific issues such as hospital exemption [4], GMO requirements for clinical trials with gene therapies [5] and a more general one that provide recommendations on these and all other aspects constituting some difficulties for the ATMP development and patient adoption [6].
This paper outlines some of issues and recommendations addressed in these papers, with a focus on hospital exemption, GMO requirements for clinical trials with gene therapies and other potential improvements in the regulatory framework, including a convergence of requirements for cells and tissues, new initiatives to simplify or facilitate regulatory processes, and to address the lack of standards in the field.
Hospital Exemption & Its Impact On ATMP Development
An important change has been introduced in the European legislation by article 28 (2) of the Regulation (EC) N°1394/2007 (so-called ATMP Regulation) amending Directive 2001/83/EC on the Community code relating to medicinal products for human use by adding paragraph 7 in article 2 of the Directive. This amendment, referred to as ‘Hospital exemption’ (HE), removes “Any advanced therapy medicinal product which is prepared on a non-routine basis according to specific quality standards, and used within the same Member State in a hospital under the exclusive professional responsibility of a medical practitioner, in order to comply with an individual medical prescription for a custom-made” from the scope of the Directive. This thus means that, when ATMPs meet the definition of HE, they may be placed on the market without a marketing authorization, provided, as further stipulated in article 2.7. of Directive 2001/83/EC, they get an authorization for manufacturing by national competent authorities and they meet national traceability, pharmacovigilance and specific quality requirements “equivalent to those provided for at Community level” for ATMPs.
The different interpretations and implementations of HE requirements in the different EU Member States have been subject to vast debate, with striking divergences of views between different stakeholders and a cause of great concern and uncertainties for industrial developers [7–10]. Examples of different implementations include the interpretation and verification of the ‘non-routine’ character for HE to be applied: Spain does not define what constitutes ‘non-routine’ use of ATMPs; France only specifies that it should be intended for small and specific populations; Italy defines it as a non-repetitive preparation; UK takes into account two factors for consideration, scale and frequency. This divergence of requirements leads to confusion and inconsistent application of the exemption scheme. There are also indications that HE may be used as a way to circumvent the applicable legal instruments for the marketing of safe and effective medicinal products in Europe, meaning it may directly delay the execution of properly executed clinical studies, compete with and even prevent market adoption of products with a marketing authorization.
Using the safeguard of public health as the guiding principle for its position, ARM has published in February 2017 a series of recommendations aiming at better defining when and how HE should be used in Europe [4]. The main recommendations were:
- To ensure that HE is limited to situations of high unmet medical need when no treatment alternatives exist; this means in practice that national competent authorities should not authorize HE when a patient can be treated for the same indication with a product with a marketing authorization of when he/she can be enrolled in an on-going clinical trial;
- To increase transparency and exchange of information by setting up a publicly available registry of all sites in Europe using HE products and mentioning in which indications each HE product is used.
In order to ensure a more harmonized and defined use of HE, ARM has proposed that the EC consider publishing guidelines to define the scope and requirements for HE. Such request for greater clarity and convergence of requirements has been further stressed in the position published a few months later by EFPIA/EBE [11].
In addition, ARM also proposed to make HE subject to the approval of an ethical committee review on a case-by-case basis to ensure that HE is the only and best possible alternative to patients and that patients are adequately informed about their treatment.
Finally, as the differences and classification between a cell, transplant, medical device, ATMP or combined ATMP may not be straightforward, meaning that physicians may actually use a product without realizing it falls under the definition of an ATMP, initiatives to increase awareness of and information for the medical community on requirements for ATMPs and to stimulate academic/industry collaboration for the development of ATMPs that meet the needs of patients would be extremely useful.
An analysis of whether ARM recommendations on HE are enforced in the existing national legislations of the largest five European Member States is provided in Table 1. For example, ARM recommendation to check the absence of alternative treatment before granting a HE does not exist in France, Germany and Spain, whilst the United Kingdom and Italy do not allow HE when a therapeutic alternative is available. In the UK, it is unclear whether a therapeutic alternative only means that an authorized product is available for the same condition or whether an alternative can also include the possible enrolment in a clinical trial with a similar type of product for the same indication. However, even in countries limiting HE in situations where there is a lack of treatment alternatives, no mechanism to periodically review the situation and renew or revoke HE exists.
| Table 1 ARM main recommendations on HE and national legal requirements for HE in the five largest EU countries. | |||||
|---|---|---|---|---|---|
| France | Germany | Italy | Spain | United Kingdom | |
| Limitation of HE in situations of high unmet medical with no available treatment alternative (authorized products) |  |  |  |  |  |
| Limitation of HE in situations of high unmet medical with no available treatment alternative (linical trials) |  |  |  |  | Unclear |
| Existence of mechanisms to periodically review the existence of treatment alternatives and treatment indications |  |  |  |  |  |
| Requirements for minimal safety and efficacy data collection on patients treated under HE |  |  |  |  |  |
| Requirement for a review by a Ethical Committee prior to granting a HE |  |  |  |  |  |
| Legal requirements in line with ARM recommendation. Legal requirements partially in line with ARM recommendation. Legal requirements not in line with ARM recommedation. | |||||
Even though HE is out of scope of what the EC can regulate (due to the way it has been introduced in the legislation), ARM firmly believes that a best practice guide developed in coordination with the EMA and NCAs would be an extremely useful tool to ensure that HE is not misused and does not constitute a disincentive for companies to invest in the development of ATMPs. It is therefore hoped that, for the benefit of patients, new initiatives along these proposals will be taken in the months to come.
GMO Requirements For Clinical Trials With Gene Therapies
Gene therapy products and some somatic cell therapy medicinal products fall under the definition of Gene Modified Organisms (GMO) and must comply with requirements of the GMO Directives [12,13] and Regulations [14,15] When clinical trials for gene therapy investigational products are conducted in Europe, such requirements come in addition to the requirements of the Clinical Trial Directive [16] until the forthcoming Regulation comes in effect in 2019 [17]. The two main directives applying to GMOs [12,13] are not specifically designed for medicinal products, raising a number of specific issues when gene therapy medicinal products need to gain approval from GMO authorities at national or regional levels. In addition, there are wide divergences in their implementation in Member States with different, competent authorities for GMO and for medicinal products. An illustration of the variability of processes and timelines in different countries is provided in Figure 1. Since requirements differ among Member States, the integration of GMO assessment in clinical trials authorization poses a challenge, particularly in the context of multicenter clinical trials, with divergent decisions and potentially inappropriate regulatory requirements for product developers.
ARM, along with EFPIA, EBE and EuropaBio believe that, in order to maintain EU competitiveness for the development of innovative ATMPs and allow patient access to these important medicines in a timely fashion, different initiatives could be taken. These proposed initiatives aim to clarify the different requirements, contacts and processes in the different Member States, streamline and shorten the overall timelines by allowing parallel assessment by the health and GMO authorities, simplify procedures by establishing a single contact point between the applicant and the relevant national authorities and foster the adoption of more uniform decisions on GMO aspects for the investigational products. Recently, the European Commission has made available on its website a repository of national regulatory requirements (EU countries and Norway) for medicinal products containing GMOs, with details of the framework, timelines, forms, languages, etc. by country [18]. This constitutes an important step forward in line with the near-term recommendations from ARM, EFPIA, EBE and EuropaBio. The regular updating of the website, however, is critical to ensure its benefit to users and national competent authorities are firmly encouraged to keep the information on this website continuously updated. It is hoped that additional measures in the near- and medium-term will be taken to allow streamlining of the assessment for clinical trials that continue to be reviewed under the Clinical Trials Directive [16], while long-term solutions need to be considered for the transition to the Clinical Trials Regulation [17].
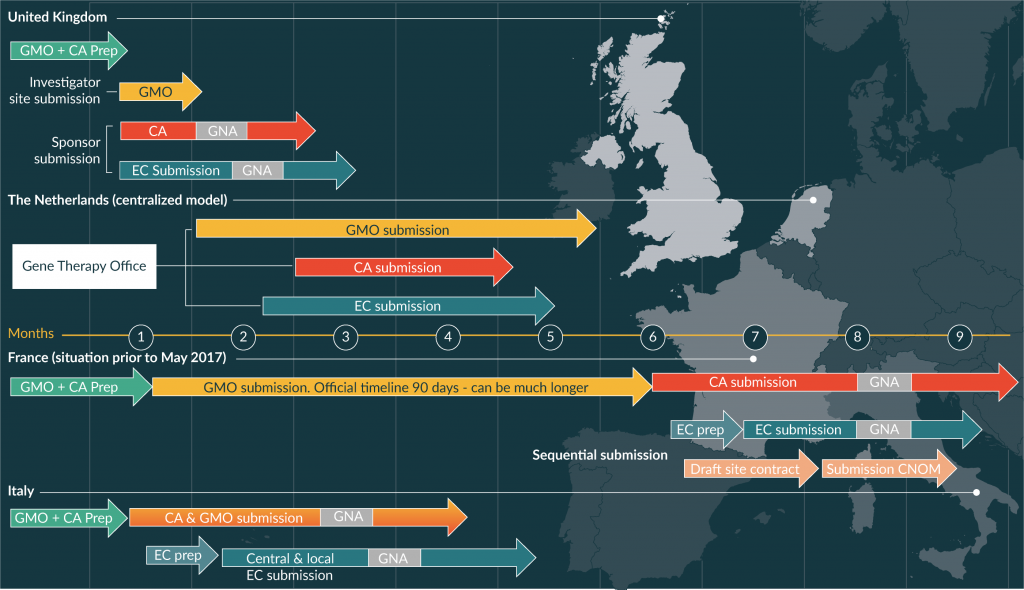
Figure 1 Examples of national processes for clinical trials application for gene therapy medicinal products. CA: Competent Health Authority; CNOM: Conseil National de l’Ordre des Médecins (French National Medical Council); EC: Ethical Committee; GMO: GMO Authority; GNA: Ground for Non-Acceptance. Note: in practice, timings can be much longer than those indicated here.
Other Potential Improvements In Regulatory Framework
Convergence Of Requirements For Cells & Tissues In Different Member States
Cells, tissues and blood are key starting materials for the products but cannot be treated as conventional starting materials because of their human origin, and the voluntary nature of the donation and the safety requirements for donors and patients to be treated with ATMPs.
Tests for donors vary greatly country by country. Wherever possible, duplication of donor testing requirements by country should be avoided and convergence of requirements on testing for cells and tissue should be reached at an international level. ARM suggests that a standard set of requirements for donor testing for tissues and cells used as starting materials for ATMP is established across all European Member States. In addition, greater transparency on whether the Cells and Tissue Directive [19] or the Blood Directive [20] should be used as the basis for some starting materials is desirable and a pragmatic approach is encouraged as already done by some Competent Authorities. Streamlining requirements for operations on human tissue and cells that fall under the scope of Directive 2004/23/EC (donation, procurement and testing of human tissues and cells) [19] or Directive 2002/98/EC (“Blood directive”) [20] and requirements for ATMP manufacturing within the same entity/facility is recommended. For instance, inspections and documentation for obtaining Tissue Establishment (TE) and Pharmaceutical establishments/GMP certifications could be aligned and to the extent possible, granted by the same authority & inspected by the same inspectorate. This would result in increased efficiency in time, documentation and manpower, both for the authorities and ATMP manufacturers. The joint and/or delegated inspection introduced in the UK is viewed as a best practice and could be implemented in other, ideally all, Member States.
Pragmatic Approach With Licensing Requirements & Regulatory Processes
Specific requirements have already been adapted in different ways to facilitate the development and the licensing requirements for ATMPs. The existence and activities of the Committee for Advanced Therapies (CAT) at the EMA and the several guidelines issued by CAT/EMA illustrates the acknowledgement of the specific expertise and different approach to be taken for ATMPs. ARM believes that new initiatives could be undertaken to further simplify regulatory requirements. These could include: workshops organised by the EMA to illustrate how the risk-based approach to determine the extent of quality, non-clinical and clinical data to be included in the marketing authorization application can be applied and used; use of a Master File system for excipients and materials used in the production of ATMPs; the implementation of a Cell History/Master File system similar to the Plasma/Vaccine Master File in the European Union; additional guidance for applicants on the IMPD and MAA structure, possibly with a draft template adapted according to the nature of the products (cell-based therapies, gene therapies, tissue-engineered products, allogeneic or autologous).
Pragmatic Approach With Licensing Requirements & Regulatory Processes
The lack of standards can be an obstacle to product development, manufacturing, evaluation and testing of advanced therapies. Even though this topic was not raised during the workshop in May 2016 and is not mentioned in the EMA report of June 2016 or the action plan in October 2017, ARM believes this is an area of priority action. This gap has been acknowledged in the U.S. Federal law enacted in 2016 required the FDA to facilitate the establishment of a Standards Coordinating Body. The Standards Coordinating Body for Regenerative Medicine (SCB) was launched in 2017. It is an independent, non-profit corporation whose mission is to work with standards developing organizations to support the development of national and international standards, to establish a Public-Private Partnership to support the development, dissemination, education and implementation of standards and to serve as a source of knowledge and experience to enable more efficient and successful clinical and commercial therapies [21].
ARM sees the need for greater engagement and coordination in Europe for the development of standards to ensure that standards are harmonized in the different regions, to improve product quality, enhance health and safety, and to strengthen market access and trade internationally. ARM encourages coordination and convergence of efforts between EU agencies or organizations such as EDQM with other international consortia such as the SCB to ensure progress and greater convergence on material and process standards essential to the timely advancement, approval and access of advanced therapies.
Conclusion
Several initiatives have already been undertaken by European regulatory authorities, in particular the European Commission and the EMA, to stimulate the development of and patients’ access to ATMPs. Such initiatives are highly valued by the sector developing ATMPs and some already proved very useful. Additional improvements to streamline requirements across the different Member States would be required to maintain European competitiveness as an investment location and a leading place for innovation. ARM has identified two areas where priority actions are required: hospital exemption and requirements for clinical trials with ATMPs with a GMO status. Additional areas of improvements with possible solutions have also been identified. It is hoped that the EC/EMA plan of actions, which require active participation and support by national competent authorities will result in concrete solutions towards greater harmonization, clarification and simplification.
ARM is very grateful to the EMA and the European Commission for their efforts to support ATMP development and market access in Europe and stands available for further contribution and collaboration with all stakeholders to make ATMP more rapidly developed and available to patients in Europe.
References
1. EMA. Advanced therapies medicines: exploring solutions to foster development and expand market access in Europe. [Online] 06 June 2016. Download PDF
2. Issues identified by stakeholders: follow-up from EMA’s ATMP workshop. [Online] 2 February 2017. Download PDF
3. European Commission-DG Health and Food Safety and EMA. European Commission – DG Health and Food Safety and European Medicines Agency Action Plan on ATMPs. [Online] October 2017. Download PDF .
4. Alliance for Regenerative Medicine. Position on Hospital Exemption. [Online] 16 February 2017. Download PDF
5. Alliance for Regenerative Medicine, et al. Possible solutions to improve the European regulatory procedures for clinical trials with Advanced Therapy Medicinal Products consisting of or containing Genetically Modified Organisms. [Online] 27 September 2017. Download PDF
6. Alliance for Regenerative Medicine. Position on possible solutions to foster development and expand patient access for Advanced Therapy Medicinal Products in Europe. [Online] 14 March 2018. Download PDF
7. Ivasiene T, Mauricas M, Ivaska J. Hospital exemption for Advanced Therapy Medicinal Products: issue in application in the European Union Member States. Curr. Stem Cell Res. Ther. 2017; 12, 45–51. CrossRef
8. Buchholz CJ, Sanzenbacher R, Schüle S. The European Hospital Exemption clause – New option for gene therapy? Hum. Gene Ther. 2012; 23, 7–12. CrossRef
9. Schnitger, Anna. The Hospital Exemption, a regulatory option for unauthorized advanced therapy medicinal products – Masters Thesis. 2004. Download PDF
10. Cuende N, Boniface C, Bravery C et al. The puzzling situation of hospital exemption for advanced therapy medicinal products in Europe and stakeholders’ concerns. Cytotherapy. 2014; 16, 1597–1600. CrossRef
11. EBE and EFPIA. Hospital Exemption for Advanced Therapy Medicinal Products (ATMPs): greater transparency needed in order to improve patient safety and access to ATMPs. [Online] 10 Oct 2017. Download PDF
12. Directive 2001/18/EC on the deliberate release of GMOs into the environment (as amended).
13. Directive 2009/41/EC on contained use of genetically modified micro-organisms.
14. Regulation (EC) 1946/2003 on transboundary movements of GMOs.
15. Regulation (EC) 1830/2009 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced by genetically modified organisms.
16. Directive 2001/20/EC of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials with medicinal products .
17. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use.
18. Genetically Modified Organism (GMO) aspects for investigational medicinal products. [Online] https://ec.europa.eu/health/human-use/advanced-therapies/gmo_investiganional_en.
19. Directive 2004/23/EC on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells.
20. Directive 2002/98/EC of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC.
21. Standards Coordinating Body, www.standardscoordinatingbody.org. [Online]
Affiliations
Annie Hubert
Alliance for Regenerative
Medicine
Jacqueline Barry
Cell & Gene Therapy Catapult
Carmen Vieira
Janssen Research & Development
Anne-Virginie Eggimann
bluebird bio
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</