Next-Generation Stem Cell Expansion Technologies
Cell Gene Therapy Insights 2018; 4(8), 791-804.
10.18609/cgti.2018.076
Cell therapies, where living cells are used as therapeutic agents, either to regenerate tissues affected by disease or to act as delivery agents for secreted factors, are commonly based on the action of stem cells or their differentiated progeny. These cells also have great potential for drug discovery, providing access to inaccessible patient-specific cell types. However, stem cells, which are the most important ‘raw material’ for these applications, have to be expanded in vitro to generate the high quantities of cells that are expected to be required. Decades of research and application of bioreactor engineering concepts have provided a solid foundation for use of advanced culture technologies for stem cell manufacturing. The particular challenges of producing high quantities of functional cells are discussed here as well as innovative approaches and technologies that may revolutionize the field.
The generation of large numbers of stem cells and derivatives is a prerequisite for the successful use of these cells for cell therapies, disease modeling and drug discovery [1–3]. As stem cell-based therapies start to enter clinical trials, the need for robust methods and consistent processes capable of providing the necessary quantities of cells in a predictable way, while ensuring the safety and functionality of the product, becomes clear [4,5]. Currently, stem cell culture is performed mostly using planar culture platforms, such as tissue culture plates and T-flasks (Figure 1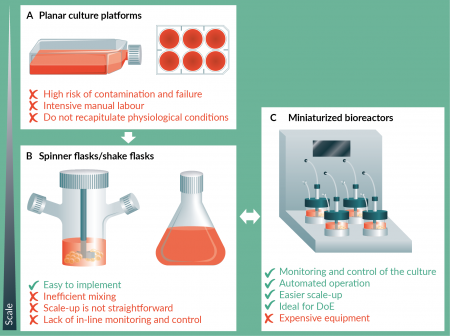
Innovative Bioreactor Systems
Stirred-tank bioreactors are the most common bioreactor type used in the biotechnology industry. These bioreactors are typically made of glass or stainless steel and are equipped with a mechanical impeller to provide agitation and to maintain cells in suspension. The culture parameters (e.g., temperature, pH, dissolved oxygen) are constantly monitored by probes installed in the vessel, and can be maintained within a working range through the action of an automated controller. Working volumes can go from milliliters, in bench-top bioreactors, to thousands of liters in large bioreactors. There are multiple examples of the application of stirred bioreactors for stem cell culture, either culturing cells on microcarriers or as aggregates [16–20].
The development of a stem cell production process starts with the optimization of culture conditions, envisaging the generation of a high cell concentration in a short time. This optimization can be achieved by using different combinations of, among others, culture media, agitation speeds, seeding densities or feeding strategies. However, a significant constraint for this optimization is the prohibitive cost of culture medium, due to the presence of expensive growth factors or cytokines, associated with a high frequency of culture medium renewal – in some cases close to daily 100% medium renewal – which limits the number of conditions to test. The mixing mechanism in stirred tank bioreactors is also not ideal for stem cells, which are more sensitive to shear stress than bacteria and, particularly in the case of cell manufacturing processes, cell integrity is fundamental for the final product quality. We will next describe innovative solutions that address these issues and that may re-invent stem cell culture in bioreactors.
Miniaturized bioreactors
A common strategy to overcome the constraints of the initial steps of process development described above consists in the use of small-scale vessels, like spinner flasks or shake flasks (Figure 1B), to minimize culture medium requirements. However, these vessels are typically not equipped with probes for culture monitoring or control and are agitated using magnetic bars, or orbital shakers, which do not mimic the impellers of higher scale bioreactors. More sophisticated, fully instrumented small-scale bioreactors have been introduced, such as the DASbox (Eppendorf) [21] or ambr 250 (Sartorius Stedim) [22] which closely mimic larger scale bioreactors, in terms of impeller geometry and control of culture conditions, allowing them to work in a 100–250 mL scale. A smaller scale system, the ambr 15 (Sartorius Stedim) [23], is also available, as a high-throughput automated workstation which allows simultaneous culture of up to 48 individually controlled vessels with working volumes as low as 10–15 mL. The possibility of testing multiple conditions in parallel in a cost-effective way (using reduced working volumes), with minimal operator-associated variability is therefore a valuable tool for process development. In fact, a process for human Mesenchymal Stem Cell (hMSC) expansion, optimized using the ambr 15, was shown to have identical performance in a higher scale [23], which confirms the validity of this approach. These systems are also particularly interesting envisaging the implementation of a Quality by Design (QbD) strategy [24], a paradigm that is changing the industrial production of biopharmaceutical products [25].
The first steps to the design of a process under QbD guidelines requires the identification of critical quality attributes (CQA) and the critical process parameters that influence them. In the case of stem cell manufacturing, it is important to identify the most important process parameters (e.g., agitation speed, seeding density, etc.) and to understand their impact on the final product, establishing the range where it is possible to operate without affecting the CQA. The use of tools like Design of Experiments (DoE) allow to run multivariate experiments, where different process parameters are varied simultaneously, elucidating the interactions between those parameters and establishing the conditions for optimal culture outcomes [26,27]. To provide statistically significant results, a high number of experimental conditions have to be tested with an appropriate number of replicates. Miniaturized bioreactor systems, like those described above, can run multiple vessels in parallel, varying and controlling the culture conditions individually, to generate data that allows to define response surfaces correlating parameter variation with quality outcomes [14]. As the process understanding increases, as well as the general stem cell biology knowledge, this rather empirical approach can be replaced with mechanistic models [24] that also correlate information about gene expression or metabolism with the CQA. Given the inherent variability associated with cellular systems, the use of automated small-scale bioreactors for process optimization allows to standardize operations performed during culture that are sources of experimental error (e.g., manual sampling or cell counting), improving the reproducibility and speeding up process development (Figure 1C).
Alternative mixing mechanisms
Stirred-tank bioreactors (Figure 2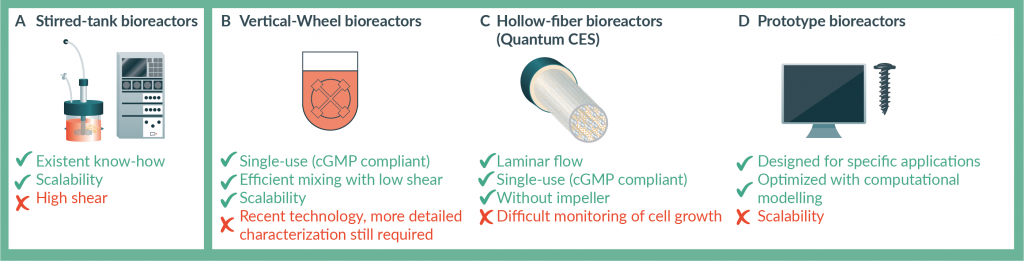
A new bioreactor type, the Vertical-Wheel bioreactor (PBS Biotech) [29], has been recently introduced, with an innovative agitation mechanism designed to promote efficient mixing with minimum power input, which can be advantageous for shear-sensitive cells (Figure 2B) [29]. This bioreactor has a U-shaped bottom and is equipped with a large vertical impeller, resulting in homogenous and gentle particle suspension, as well as a high mass transfer rate, under low agitation speeds. The Vertical-Wheel bioreactors were developed as single-use vessels, envisaging compliance with current Good Manufacturing Practices (cGMP), and are available in different volumetric scales. Miniaturized versions of these reactors (100–500 mL of working volume) can be used, as described above, for process development – although these vessels are not instrumented. Above the 3 L model, and up to 80 L, the bioreactors allow automated control of the culture and consistent mixing performance across the scales. However, since the Vertical-Wheel bioreactor technology is relatively new, more detailed engineering studies regarding fluid dynamics or mass transfer are still necessary for the complete characterization of the system. Nevertheless, successful use of this bioreactor for hMSC [30] and hiPSC [31] expansion using microcarriers has been reported and thus this should be also a promising technology for stem cell culture using other formats or cell types.
Stirred-tank bioreactors and Vertical-Wheel bioreactors generate fluid motion by the action of an impeller that, through its rotation, provides the dynamic environment that enhances culture medium mixing and particle suspension. However, other bioreactor geometries are available which generate mixing without a mechanical impeller. Hollow-fiber bioreactors [32], where anchorage-dependent cells are cultured attached to the fibers while culture medium is perfused continuously, in a way that resembles the human circulatory system, are a low-shear stress alternative to stirred bioreactors (Figure 2C). The Quantum Cell Expansion System (Terumo BCT) is a closed-system, automated hollow-fiber bioreactor that has been recently used for the expansion of hMSC [33,34] as well as neural stem cells (NSC) [35]. These studies present promising results but aspects such as monitoring of cell morphology or growth are still not straightforward with hollow-fiber bioreactors and can be the object of future research.
An interesting alternative geometry, conceived specifically to provide a laminar flow regime, while avoiding the presence of impellers/rotating components, was described by Massai and colleagues [36]. The bioreactor consists of a stainless steel base, a culture chamber and a lid. The continuous and closed-loop circulation of medium in the culture chamber leads to the generation of buoyant vortices for particle (e.g., microcarriers or cell aggregates) suspension under low-to-moderate shear stress, depending on the flow rate. The shape of the culture chamber was conceived and further improved using in silico models, in order to achieve optimized performance. Although the culture of stem cells was not reported, the authors show proof-of-concept of the application of this bioreactor with cancer cell spheroids. This study illustrates the design of innovative prototype bioreactor configurations for mammalian cell culture (Figure 2D), through computational modeling, prototyping and experimental validation, which may be an interesting solution, in particular for stem cell differentiation bioprocesses that may require tunable levels of shear to maintain cell viability but also, in some cases, to stimulate differentiation [37].
New strategies & technologies for stem cell culture
The bioreactor configurations described above have the potential to take stem cell manufacturing to a next level. Nevertheless, new approaches and strategies for improved cell culture performance combined with cutting-edge technologies for monitoring of the culture parameters and also for cell recovery/downstream processing are equally important. In this section, we will discuss the benefits and bottlenecks of different culture formats (microcarriers vs cell aggregates) and review how recent scientific and technological advances may lead to the development of ‘advanced microcarriers’, to alternative culture medium feeding strategies (perfusion) and also to new devices for culture monitoring that may impact the field of stem cell bioprocessing (Figure 3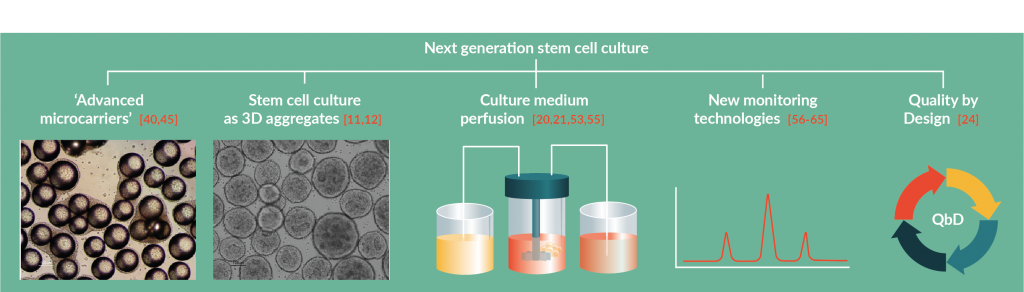
Development of ‘advanced microcarriers’
The transition of stem cell culture from static 2D plates to a 3D format is often performed using microcarriers, which are particles (100–300 µm of diameter) to which cells adhere and grow, usually coated with extracellular matrix substrates [38]. The surface-to-volume ratio of microcarriers is much higher than in planar platforms and the available surface area for the cells to grow can easily be increased by adding more microcarriers. Keeping the microcarriers in suspension in stirred bioreactors requires high agitation speeds, which may damage the cells not only directly by the shear forces but also due to the bead-to-bead collisions. Also, the cells have to be harvested from the microcarriers at the end of the culture. Indeed, the most commonly used microcarriers are polystyrene microspheres and, to meet the regulatory and safety demands for the presence of particulates, the microcarriers have to be efficiently removed from the final product [39]. The subsequent use of the cells expanded using this method therefore requires two downstream operations: detaching the cells from the microcarriers and a separation step to remove the microcarriers from the cell suspension. Innovative approaches have been reported to address these challenging stages. Similarly to what happens in culture plates, proteolytic enzymes are frequently used to detach cells from microcarriers. However, the action of these enzymes may be detrimental for cell viability. The use of microcarriers made, or coated, with stimuli-responsive biomaterials is a promising alternative strategy [40]. Stimuli-responsive materials change their chemical or physical properties in response to external environmental changes. The coating of microcarriers with poly N-isopropylacrylamide (pNIPAAm), a thermoresponsive polymer [41], allows cell detachment by decreasing the temperature under 32 °C, allowing to harvest the cells without proteases which may damage cell membrane proteins [42,43]. This approach has been successfully reported in the literature with Chinese Hamster Ovary (CHO) cells [44] and also with hMSC [45]. Polymers sensitive to other stimuli like pH, light, ultrasounds and electric or magnetic fields are also available with interesting properties for microcarrier production [40] but their use with stem cells has not been reported. However, although more efficient cell detachment can be obtained using these polymers, it remains necessary to remove the microcarriers from the final product. This separation process can be performed by filtration using cell strainers, in small-scale processes, or using, for instance, tangential flow filtration systems, when moving to larger scales [39]. These operations may result in cell losses/decreased viability and increase the complexity of the process. The production of microcarriers made of biomaterials, such as hydrogels that can be dissolved enzymatically or by other means, with minimal harm to the cells, may also bring many advantages to the downstream processing stage and a commercial product with these characteristics has recently been introduced by Corning. Besides these bioprocessing aspects, the manipulation of the physical properties of the microcarriers is also interesting to modulate stem cell fate. Many studies describe the impact of mechanical cues on stem cell proliferation and differentiation (reviewed in [46]) and the use of microcarriers made of materials with different properties in terms of elasticity or topography may be a promising strategy for stem cell manufacturing [47]. Moreover, the microcarriers can be used as agents for controlled delivery of growth factors or small molecules, enhancing, for instance, differentiation efficiency [48].
Stem cell culture as 3D aggregates
A viable alternative to the use of microcarriers is growing the cells as self-organized spherical aggregates [11,12]. In contrast to microcarrier-based culture there is no need for external particles and/or matrices and, importantly, at the end of culture, the cells can be harvested directly, without the need to be separated from the particles, simplifying the process and avoiding cell losses. Stem cell aggregates can also be used to generate organoids, which are cellular structures that replicate, in a simplified way, the structural and functional features of a specific organ [49].
Despite these advantages, aggregate culture may present limitations in terms of diffusion to the centre of the aggregates when they grow above 200–300 µm [50] and heterogeneity in aggregate size may lead to inconsistent results. These problems can be avoided if aggregate size is controlled either through frequent passaging (every 3–4 days), by adjusting the stirring rate or by chemical-based methods, as described in a recent publication [51]. Although aggregate culture is sometimes difficult to implement, in particular using bioreactors, encouraging advances have been recently reported either with hMSC [52] or hiPSC [21,53] and, in the future, interesting developments are expected regarding organoid culture in bioreactors [49,54].
Culture medium perfusion
In most of reported cases of stem cell culture in bioreactors, culture medium feeding is performed, similarly to what happens in static culture, using the so-called ‘repeated batch’ scheme [6], which consists of replacing a fraction or the whole volume of culture medium in regular time intervals. This methodology, however, may lead to significant oscillations in culture medium composition (e.g., nutrients, toxic metabolites, cell-secreted factors) and also in terms of pH or dissolved oxygen. Recent studies demonstrate the benefit of using perfusion of culture medium as a way to improve stem cell expansion [20,21,53,55]. Perfusion consists in the continuous addition of fresh medium and removal of spent medium while the cells are retained inside the bioreactor, leading to a more stable culture environment. However, while some bioreactor configurations depend on perfusion to operate (e.g., hollow-fiber bioreactors), the implementation of this feeding strategy with other bioreactors may be technically demanding, requiring cell retention devices and the availability of feeding and harvesting ports, which can be particularly complex when using simple devices, such as spinner flasks. However, the culture of hiPSC in instrumented small-scale stirred bioreactors using perfusion was implemented and reported to result in a 47% higher cell yield when compared with repeated batch [21], highlighting the advantage of adopting this more physiological feeding strategy, which may be an additional way to better mimic the in vivo stem cell microenvironment.
Monitoring technology
As mentioned above, bioreactors are equipped with probes that allow monitoring of the culture environment. However, the probes used are mostly able to measure conventional physicochemical factors (e.g., temperature, pH, dissolved oxygen) and therefore, the ability to measure other more specific culture parameters (concentration of glucose or lactate and cell density) or even to characterize the cell population (e.g., undifferentiated stem cells vs differentiated cells) in real time are not frequently used – or not available at all – and may be important to further improve the culture performance and reduce batch-to-batch variability.
The concentration of nutrients (e.g., glucose) and metabolites (e.g., lactate) during bioreactor culture is often measured off-line and the results are not used for automated feedback control of the culture. Methods for measurement of glucose or lactate concentrations, using spectroscopic analysis [56] are available and, as an example, mid-infrared (MIR) spectroscopy has been used to analyze the supernatants of hMSC cultures on microcarriers [57] and could be in the future used for real-time optimization of medium feeding to bioreactors. Other spectroscopic methods such as Raman or 2D-fluorescence were also used for the same purposes with mammalian cell lines and could, in principle, be also used with stem cells [58].
The possibility to have online measurement of other stem cell specific parameters would greatly increase the knowledge about the process and potentially facilitate the adoption of QbD [24]. A method for tracking stem cell growth on microcarriers, using an optical system, has been developed for automated and operator-independent measurement of cell viability, confluence and cell distribution [59]. Dielectric spectroscopy has also been used for viable cell quantification on microcarriers with encouraging results [60]. The characterization of the cells generated in the process, however, involves assays that often require sacrificing cell samples and lengthy protocols, such as transcriptional/proteomic profiling or functional assays (e.g., differentiation potential, in vivo transplantation), which are not suitable for real-time monitoring of bioprocesses. Alternative assays that can give indications of cell identity/quality in real time are therefore necessary. Raman spectroscopy has been used to non-invasively measure molecular properties of stem cells during differentiation in vitro [61] as this label-free technique allows to monitor time-dependent molecular changes in live cells, providing information about cell phenotype, without affecting cell viability. Examples of the use of Raman spectroscopy include the ability to identify and distinguish NSC and NSC-derived glial cells [62], monitoring cardiac differentiation of human embryonic stem cells [63] and, more recently, for the discrimination of atrial and ventricular cardiomyocytes derived from pluripotent stem cells [64]. The possibility to integrate these techniques in bioreactor systems will be a huge step forward towards the manufacturing of high-quality stem cell-based products. Although online monitoring of stem cell/differentiated progeny properties is not yet possible, the use of Raman spectroscopy for evaluation of the maturity of cardiomyocytes derived from hiPSC in bioreactors has been described [65] and in forthcoming years we may see the emergence of groundbreaking devices for stem cell bioprocess monitoring.
Translational insight
The success of stem cell-based therapies or, in a shorter-term, the use of stem cells in drug discovery and/or toxicity evaluation, will greatly depend on the availability of technologies for efficient and reliable cell manufacturing. Recent advances are opening new doors that will be crucial to overcome many challenges that still exist in the field. Currently, many steps of process optimization are performed in small-scale culture vessels, which are difficult to monitor and control. Miniaturized bioreactors, as discussed above, allow the performance of high-throughput experiments, in order to establish the optimal ranges of culture parameters, in a vessel geometry that can be translated to larger scale bioreactors. The use of these bioreactors also may lead to more reproducible experimental outcomes and the detailed process data collected can be used to implement QbD strategies [24]. The availability of online monitoring technologies, for important parameters for stem cell culture, is expected to lead, as well, to tighter control of the bioreactor culture performance and to improved outcomes. Agitation/mixing paradigms are also evolving to take into account the requirements of stem cell cultivation. Traditional stirred bioreactors have been used for stem cell culture but there are concerns about the possibility of shear-induced cell death or differentiation [66]. Different strategies have been used to design bioreactors with low-shear environments and it is predicted that new bioreactor geometries (as illustrated in [36]) can be introduced in the future using computational fluid dynamics modeling and the current availability of 3D printing equipment may facilitate even more the construction of new prototypes that can after be validated experimentally.
The inventive combination of some of the different bioprocessing approaches here described may also be a way of targeting culture challenges, as exemplified in a recent work where hMSC aggregates were obtained by combining pre-expansion on pNIPAAm-coated microcarriers, followed by temperature-induced cell detachment and aggregate formation using Vertical-Wheel bioreactors [67]. Most of the current bioreactor technology has been developed envisaging the production of high concentrations of proteins and not high concentrations of functional, living cells. The methodologies here described, including the use of new bioreactors, different culture modes (‘advanced microcarriers’, aggregates), feeding strategies (perfusion) or monitoring devices, were already able to generate relatively high cell densities (Table 1). Further developments of these technologies, together with new fundamental biology knowledge, will allow the design of new stem cell-oriented bioprocesses, which will take the field into the next generation of stem cell culture.
| Summary of maximum cell quantities obtained with different bioreactors and stem cell types. | ||||
|---|---|---|---|---|
| Bioreactor | Cell type | Maximum cell density (cells/mL) | Total cells harvested | Ref. |
| DASbox (w/ perfusion) | hiPSC | 2.85 ± 0.34 x106 | 3.56 ± 0.43 x108 | [21] |
| ambr 15 | hMSC | 1.51 ± 0.06 x105 | 2.27 ± 0.09 x106 | [23] |
| Vertical-Wheel (PBS 3) | hMSC | 3 x105 | 6.6 x108 | [30] |
| Vertical-Wheel (PBS 0.1) | hiPSC | 1.21 ± 0.02 x106 | 9.68 ± 0.16 x107 | [31] |
| Quantum CES | Human periosteum-derived stem cells | N/A | 3.71 x108 | [33] |
| Quantum CES | hMSC | N/A | 2.40 x108 | [34] |
| Quantum CES | hNSC | N/A | 2-3 x108 | [35] |
| Spinner flask (w/ pNIPAAm microcarriers) | hMSC | ~5 x105 | ~ 1.5 x106 | [45] |
| Stirred bioreactor (w/ perfusion) | hiPSC | 3.9 ± 0.2 x106 | 7.8 ± 0.4 x108 | [53] |
| Vertical-Wheel (PBS 0.1 w/pNIPAAm microcarriers) | hMSC | ~1.1 x105 | ~ 6.6 x106 | [67] |
| N/A: Not applicable. | ||||
Financial & competing interests disclosure
The authors acknowledge financial support from Fundação para a Ciência e a Tecnologia (FCT), Portugal and from Programa Operacional Regional de Lisboa 2020, through iBB – Institute for Bioengineering and Biosciences (UID/BIO/04565/2013; Project N. 007317), through the project PRECISE (PAC – PRECISE-LISBOA-01-0145-FEDER-016394, SAICTPAC/0021/2015) and the projects CEREBEX (PTDC/BTM-SAL/29298/2017, LISBOA-01-0145-FEDER-029298) and CARDIOWHEEL (PTDC/EQU-EQU/29653/2017). Also, the European Union Framework Programme for Research and Innovation HORIZON 2020, under the TEAMING Grant agreement No 739572 (H2020-WIDESPREAD-01-2016-2017) – The Discoveries CTR. Diogo E.S. Nogueira thanks FCT for financial support (PD/BD/128376/2017).
References
1. Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016; 17(3): 170–82. CrossRef
2. de Soure AM, Fernandes-Platzgummer A, da Silva CL, Cabral JM. Scalable microcarrier-based manufacturing of mesenchymal stem/stromal cells. J. Biotechnol. 2016; 236: 88–109. CrossRef
3. Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. 2016; 17(3): 194–200. CrossRef
4. Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell 2008; 3(4): 369–81. CrossRef
5. Rodrigues CA, Fernandes TG, Diogo MM, da Silva CL, Cabral JM. Stem cell cultivation in bioreactors. Biotechnol. Adv. 2011; 29(6): 815–29. CrossRef
6. Kropp C, Massai D, Zweigerdt R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem. 2017; 59: 244–54. CrossRef
7. Rafiq QA, Coopman K, Hewitt CJ. Scale-up of human mesenchymal stem cell culture: current technologies and future challenges. Curr. Opin. Chem. Eng. 2013; 2(1): 8–16. CrossRef
8. Archibald PR, Chandra A, Thomas D et al. Comparability of automated human induced pluripotent stem cell culture: a pilot study. Bioprocess.
Biosyst. Eng. 2016; 39(12): 1847–58. CrossRef
9. Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012; 125(Pt 13): 3015–24. CrossRef
10. Badenes SM, Fernandes TG, Rodrigues CAV, Diogo MM, Cabral JMS. Microcarrier-based platforms for in vitro expansion and differentiation of human pluripotent stem cells in bioreactor culture systems. J. Biotechnol. 2016; 234: 71–82. CrossRef
11. Egger D, Tripisciano C, Weber V, Dominici M, Kasper C. Dynamic Cultivation of Mesenchymal Stem Cell Aggregates. Bioengineering (Basel) 2018; 5(2): pii: E48.
12. Sart S, Bejoy J, Li Y. Characterization of 3D pluripotent stem cell aggregates and the impact of their properties on bioprocessing. Proc.
Biochem. 2017; 59: 276–88. CrossRef
13. Fernandes TG, Rodrigues CAV, Diogo MM, Cabral JMS. Stem cell bioprocessing for regenerative medicine. J. Chem. Technol. Biotechnol. 2014; 89(1): 34–47. CrossRef
14. Campbell A, Brieva T, Raviv L et al. Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl. Med. 2015l 4(10): 1155–63. CrossRef
15. Eaker S et al. Bioreactors for cell therapies: Current status and future advances. Cytotherapy 2017; 19(1): 9–18. CrossRef
16. Dos Santos F, Campbell A, Fernandes-Platzgummer A et al. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol. Bioeng, 2014. 111(6): 1116–27. CrossRef
17. Fernandes-Platzgummer A, Diogo MM, Baptista RP, da Silva CL, Cabral JM. Scale-up of mouse embryonic stem cell expansion in stirred bioreactors. Biotechnol. Prog. 2011; 27(5): 1421–32. CrossRef
18. Rafiq QA, Brosnan KM, Coopman K, Nienow AW, Hewitt CJ. Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett. 2013; 35(8): 1233–45. CrossRef
19. Schroeder M, Niebruegge S, Werner A et al. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol. Bioeng. 2005; 92(7): 920–33. CrossRef
20. Serra M, Brito C, Sousa MF et al. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J. Biotechnol. 2010; 148(4): 208–15. CrossRef
21. Kropp C, Kempf H, Halloin C et al. Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Stem Cells Transl. Med. 2016; 5(10): 1289–301. CrossRef
22. Xu P, Clark C, Ryder T et al. Characterization of TAP Ambr 250 disposable bioreactors, as a reliable scale-down model for biologics process development. Biotechnol. Prog. 2017; 33(2): 478–89. CrossRef
23. Rafiq QA, Hanga MP, Heathman TRJ et al. Process development of human multipotent stromal cell microcarrier culture using an automated high-throughput microbioreactor. Biotechnol. Bioeng. 2017. 114(10): 2253–66. CrossRef
24. Lipsitz YY, Timmins NE, Zandstra PW. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016; 34(4): 393–400. CrossRef
25. Rathore AS, Winkle H. Quality by design for biopharmaceuticals. Nat. Biotechnol. 2009; 27(1): 26–34. CrossRef
26. Badenes SM, Fernandes TG, Cordeiro CS et al. Defined Essential 8 Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS One 2016; 11(3): e0151264. CrossRef
27. Hunt MM, Meng G, Rancourt DE, Gates ID, Kallos MS. Factorial experimental design for the culture of human embryonic stem cells as aggregates in stirred suspension bioreactors reveals the potential for interaction effects between bioprocess parameters. Tissue Eng. Part C Methods 2014; 20(1): 76–89. CrossRef
28. Fridley KM, Kinney MA, McDevitt TC. Hydrodynamic modulation of pluripotent stem cells. Stem Cell Res. Ther. 2012; 3(6): 45. CrossRef
29. Croughan MS, Giroux D, Fang D, Lee B. Novel Single-Use Bioreactors for Scale-Up of Anchorage-Dependent Cell Manufacturing for Cell Therapies in Stem Cell Manufacturing. da Silva CL, Chase LG, Diogo MM (Eds). 2016, Elsevier. 105–39.
30. Sousa MF, Silva MM, Giroux D et al. Production of oncolytic adenovirus and human mesenchymal stem cells in a single-use, Vertical-Wheel bioreactor system: Impact of bioreactor design on performance of microcarrier-based cell culture processes. Biotechnol. Prog. 2015; 31(6): 1600–12. CrossRef
31. Rodrigues CAV, Silva TP, Nogueira DES et al. Scalable Culture Of Human Induced Pluripotent Cells On Microcarriers Under Xeno-Free Conditions Using Single-Use Vertical-Wheel™ Bioreactors. J. Chem. Technol. Biotechnol. 2018; doi:10.1002/jctb.5738. CrossRef
32. Piret JM, Cooney CL. Immobilized mammalian cell cultivation in hollow fiber bioreactors. Biotechnol. Adv. 1990; 8(4): 763–83. CrossRef
33. Lambrechts T, Papantoniou I, Rice B, Schrooten J, Luyten FP, Aerts JM. Large-scale progenitor cell expansion for multiple donors in a monitored hollow fibre bioreactor. Cytotherapy 2016; 18(9): 1219–33. CrossRef
34. Mizukami A, de Abreu Neto MS, Moreira F et al. A Fully-Closed and Automated Hollow Fiber Bioreactor for Clinical-Grade Manufacturing of Human Mesenchymal Stem/Stromal Cells. Stem Cell Rev. 2018; 14(1): 141–3. CrossRef
35. Tirughana R, Metz MZ, Li Z et al. GMP Production and Scale-Up of Adherent Neural Stem Cells with a Quantum Cell Expansion System. Mol. Ther. Methods Clin. Dev. 2018; 10: 48–56. CrossRef
36. Massai D, Isu G, Madeddu D et al. A Versatile Bioreactor for Dynamic Suspension Cell Culture. Application to the Culture of Cancer Cell Spheroids. PLoS One 2016; 11(5): e0154610. CrossRef
37. Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol. Bioeng. 2013; 110(4): 1231–42. CrossRef
38. Chen AK, Reuveny S, Oh SK. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotechnol. Adv. 2013; 31(7): 1032–46. CrossRef
39. Schnitzler AC, Verma A, Kehoe DE et al. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem. Eng. J. 2016; 108: 3–13. CrossRef
40. Tavassoli H. Large-scale Production of Stem Cells Utilizing Microcarriers: A Biomaterials Engineering Perspective from Academic Research to Commercialized Products. Biomaterials 2018; doi:10.1016/j.biomaterials.2018.07.016. CrossRef
41. Gandhi A, Paul A, Sen SO, Sen KK. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015; 10(2): 99–107. CrossRef
42. Huang HL, Hsing HW, Lai TC et al., Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 2010; 17: 36. CrossRef
43. Yamato M. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polymer Sci. 2007; 32(8): 1123–1133. CrossRef
44. Tamura A, Kobayashi J, Yamato M, Okano T. Temperature-responsive poly(N-isopropylacrylamide)-grafted microcarriers for large-scale non-invasive harvest of anchorage-dependent cells. Biomaterials 2012; 33(15): 3803–12. CrossRef
45. Yang HS, Jeon O, Bhang SH, Lee SH, Kim BS. Suspension culture of mammalian cells using thermosensitive microcarrier that allows cell detachment without proteolytic enzyme treatment. Cell Transplant 2010; 19(9): 1123–32. CrossRef
46. Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017; 18(12): 728–42. CrossRef
47. Abdeen AA, Saha K. Manufacturing Cell Therapies Using Engineered Biomaterials. Trends Biotechnol. 2017; 35(10): 971–82. CrossRef
48. Soup ST, Shin-Hyun K, Seung-Man Y. Elaborate Design Strategies Toward Novel Microcarriers for Controlled Encapsulation and Release. Part. Part. Syst. Character. 2013; 30(1): 9–45. CrossRef
49. Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat. Rev. Genet. 2018; doi:10.1038/s41576-018-0051-9. CrossRef
50. Wu J, Rostami MR, Cadavid Olaya DP, Tzanakakis ES. Oxygen transport and stem cell aggregation in stirred-suspension bioreactor cultures. PLoS One 2014; 9(7): p. e102486. CrossRef
51. Lipsitz YY, Tonge PD, Zandstra PW. Chemically controlled aggregation of pluripotent stem cells. Biotechnol. Bioeng. 2018; 115(8): 2061–6. CrossRef
52. Tsai AC, Liu Y, Yuan X, Chella R, Ma T. Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnol. J. 2017; 12(5), doi:10.1002/biot.201600448. CrossRef
53. Abecasis B, Aguiar T, Arnault É et al. Expansion of 3D human induced pluripotent stem cell aggregates in bioreactors: Bioprocess intensification and scaling-up approaches. J. Biotechnol. 2017; 246: 81–93. CrossRef
54. Przepiorski A, Sander V, Tran T et al. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Reports 2018; 11(2): 470–84. CrossRef
55. Fernandes-Platzgummer A, Diogo MM, Lobato da Silva C, Cabral JMS. Maximizing mouse embryonic stem cell production in a stirred tank reactor by controlling dissolved oxygen concentration and continuous perfusion operation. Biochem. Eng. J. 2014; 82: 81–90. CrossRef
56. Teixeira AP, Oliveira R, Alves PM, Carrondo MJ. Advances in on-line monitoring and control of mammalian cell cultures: Supporting the PAT initiative. Biotechnol. Adv. 2009; 27(6): 726–32. CrossRef
57. Rosa F, Sales KC, Carmelo JG et al. Monitoring the ex-vivo expansion of human mesenchymal stem/stromal cells in xeno-free microcarrier-based reactor systems by MIR spectroscopy. Biotechnol. Prog. 2016; 32(2): 447–55. CrossRef
58. Rowland-Jones RC, van den Berg F, Racher AJ, Martin EB, Jaques C. Comparison of spectroscopy technologies for improved monitoring of cell culture processes in miniature bioreactors. Biotechnol. Prog. 2017; 33(2): 337–46. CrossRef
59. Odeleye AOO, Castillo-Avila S, Boon M, Martin H, Coopman K. Development of an optical system for the non-invasive tracking of stem cell growth on microcarriers. Biotechnol. Bioeng. 2017; 114(9): 2032–42. CrossRef
60. Justice C, Leber J, Freimark D et al. Online- and offline- monitoring of stem cell expansion on microcarrier. Cytotechnology 2011; 63(4): 325–35. CrossRef
61. Ghita A, Pascut FC, Sottile V, Denning C, Notingher I. Applications of Raman micro-spectroscopy to stem cell technology: label-free molecular discrimination and monitoring cell differentiation. EPJ Tech. Instrum. 2015; 2(1): 6. CrossRef
62. Ghita A, Pascut FC, Mather M, Sottile V, Notingher I. Cytoplasmic RNA in undifferentiated neural stem cells: a potential label-free Raman spectral marker for assessing the undifferentiated status. Anal. Chem. 2012; 84(7): 3155–62. CrossRef
63. Pascut FC, Goh HT, Welch N et al. Noninvasive detection and imaging of molecular markers in live cardiomyocytes derived from human embryonic stem cells. Biophys. J. 2011; 100(1): 251–9. CrossRef
64. Brauchle E, Knopf A, Bauer H et al. Non-invasive Chamber-Specific Identification of Cardiomyocytes in Differentiating Pluripotent Stem Cells. Stem Cell Rep. 2016; 6(2): 188–99. CrossRef
65. Shen N, Knopf A, Westendorf C et al. Steps toward Maturation of Embryonic Stem Cell-Derived Cardiomyocytes by Defined Physical Signals. Stem Cell Rep. 2017; 9(1): 122–35. CrossRef
66. Leung HW, Chen A, Choo AB, Reuveny S, Oh SK. Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng. Part C Methods 2011; 17(2): 165–72. CrossRef
67. Yuan X, Tsai AC, Farrance I, Rowley JA, Ma T. Aggregation of culture expanded human mesenchymal stem cells in microcarrier-based bioreactor. Biochem. Eng. J. 2018. 131: 39-46. CrossRef

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.