‘Winning’ target product profiles for CAR-T cell therapies in oncology: critical success factors for commercially viable therapies
Cell & Gene Therapy Insights 2024; 10(11), 1629–1643
DOI: 10.18609/cgti.2024.189
The global CAR-T cell therapy market is predicted to reach US$29.0 billion in revenues by 2029, growing at a CAGR rate of 39.6% from 2024–2029. Despite the attractiveness of the potential market, CAR-T cell therapies have high costs of goods (COGs) due to their complex manufacturing process and a high price, making it crucial for CAR-T cell developers to develop a target product profile (TPP) at an early stage to determine if a commercially attractive product is achievable and hence viable. This article emphasizes the importance of developing a TPP for CAR-T cell therapies and offers best practice guidance on its development. It highlights the strategic role of a TPP in clinical development and the value of adopting an evidence-based approach to developing a TPP, as led by a cross-functional multi-disciplinary team. A CAR-T-specific template is proposed, combining various TPP templates. Finally, we provide clarity on what a ‘good’ TPP looks like, optimal timing for development, governance processes, data and insights needed, and key ‘watch outs’.
Since 2017, cell therapies have been the focus of immuno-oncology development [1]Yu JX, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019; 18(12), 899–900., with over 800 CAR-T cell therapy clinical studies in progress globally (as of September 9, 2024 [2]National Institutes for Health. Clinicaltrials.gov search results. (accessed Sep 5, 2024). ). The potential for further growth in this sector is evident, with the majority of ongoing studies still in the early stages of clinical development (Figure 1). From a commercial perspective, market analysis predicts the global CAR-T cell therapy market could reach US$29.0 billion in revenues by 2029, growing at a CAGR rate of 39.6% from 2024–2029 [3]Markets and Markets. CAR-T cell therapy market size, share and trends. cell-therapy-market-47772841.html#:~:text=The%20size%20of%20global%20CAR,39.6%25%20from%202024%20to%202029 (accessed Sep 9, 2024)..
R&D expenditure on CAR-T cell therapies is not often publicly disclosed. However, it is well understood that due to the complex manufacturing process, CAR-T cell treatments incur high COGs that surpass other cancer therapies [4]Stanton D. Cost of goods is crucial for the future of regenerative medicine: CAR-T cell therapy provides a case study in perspective. BioProcess International Apr 18, 2019. (accessed Sep 9, 2024).. The COGs, together with the hefty investment in undertaking clinical trials and developing a scalable manufacturing infrastructure, mean that it is imperative that CAR-T cell developers have a robust understanding of what constitutes a commercially viable product to yield a return. The latter can be greatly facilitated through the development of a TPP that pre-specifies the attributes of treatment most likely to lead to a regulatory approvable, reimbursable treatment that is highly valued by patients.
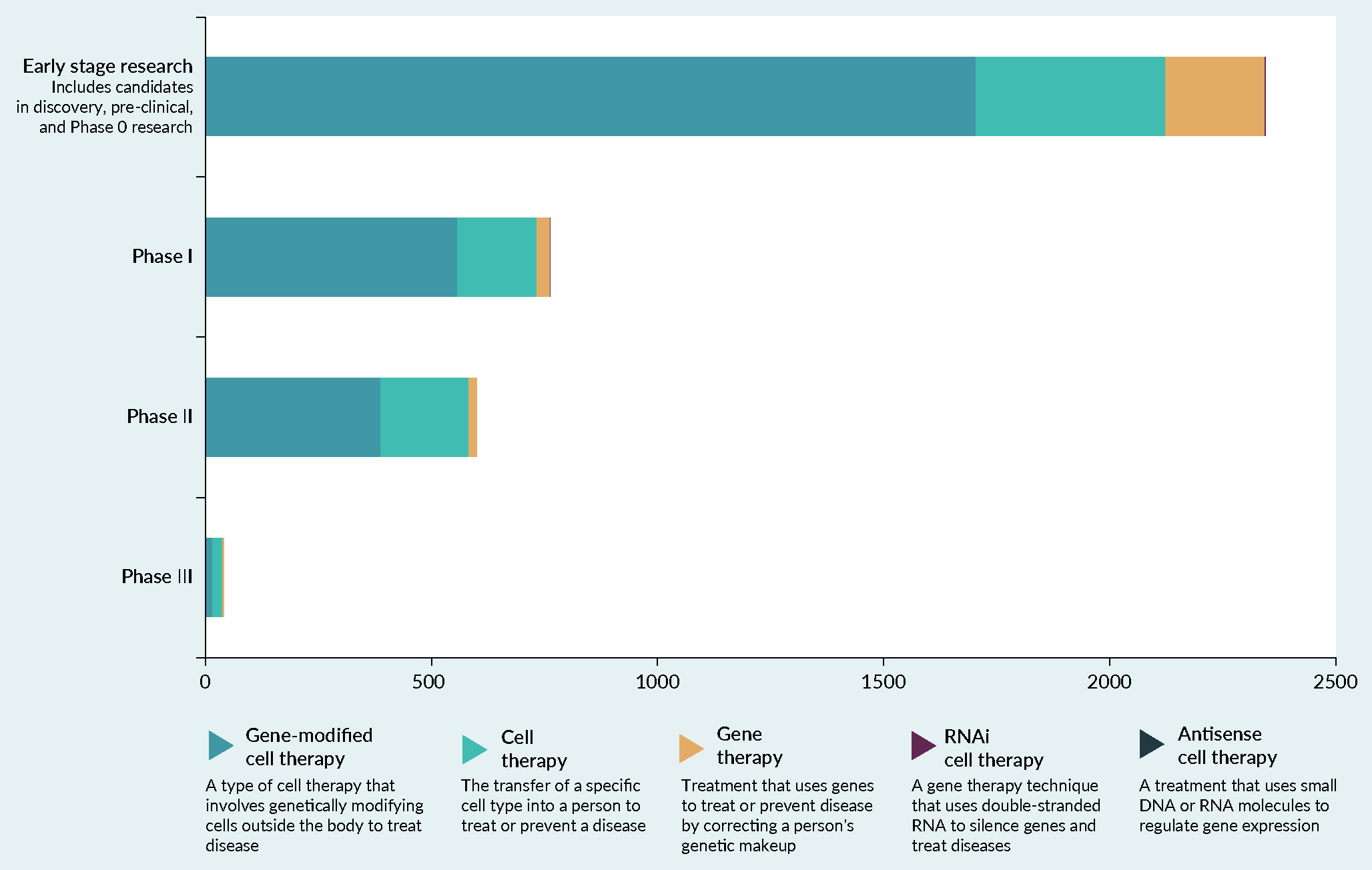
Figure 1. The number of CAR-T cell therapies under clinical development by phase of development and disease. |
|---|
Adapted from [30]. Data not visible: 3× RNAi gene therapies in early stage development and 1× antisense gene therapy in early stage development. |
The aims of this article are to raise awareness amongst CAR-T cell developers of the importance of developing a TPP at an early stage of product development and to offer best practice guidance on how to go about pulling a TPP together.
First, we outline what we understand to be a TPP based on a synthesis of the published and grey literature, as well as the strategically beneficial role that a TPP can play in clinical development.
Second, we describe how to best go about developing a TPP emphasizing the value of CAR-T cell developers in charging a cross-functional multi-disciplinary team from within their organization with the task of developing this, so that an internally aligned initial draft can be externally validated by patient experts, clinicians, regulators, HTA agencies, and payers. An adapted version of a CAR-T cell specific template is then proposed, which is an amalgamation of different TPP templates reported elsewhere.
Third, we seek to provide some clarity around what might be considered a ‘good’ TPP whilst providing some recommendations around when a TPP should be developed as well as the frequency at which it should be updated and how the TPP process could best be governed.
Finally, we cover which data and insights are needed to develop a TPP for a CAR-T cell therapy together with the key uncertainties and ‘watch outs’.
What is a target product profile (TPP) and what is its purpose?
A TPP can best be described as a strategic planning tool that lays out a priori the desired profile of a new therapy that, if realized, can ensure a reasonable probability of regulatory and market access success [5]US Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry and Review Staff Target Product Profile—A Strategic Development Process Tool. 2007. (accessed Sep 5, 2024). [6]US Food and Drug Administration; Center for Drug Evaluation Research; Center for Biologics Evaluation Research. Guidance for Industry. Q8(R2) Pharmaceutical Development. Nov 2009. (accessed Sep 5, 2024).. It includes, but is not limited to, the target indication(s), target patient population(s), and important safety/efficacy/patient-reported outcome (PRO) and health economic characteristics and parameters [7]National Institutes of Health; SEED. Creating a Target Product Profile for New Drug Products. (accessed Sep 9, 2024).. 20 years ago, a TPP might have focused on setting out the minimum requirements for regulatory approvable treatments. However, it can no longer be assumed that regulatory success equates to commercial success. This is because following regulatory approval in Europe, CAR-T cell developers need to clear an additional hurdle, i.e., secure reimbursement from Health Technology Assessment (HTA) agencies and payers in different countries which has proven more challenging. The evidence gap between the regulatory requirements with the more stringent HTA and payer requirements is what the TPP needs to mitigate against, and as such is why we consider that broader considerations such as market positioning, differentiation and cost-effectiveness (amongst others) should not be treated separately from the TPP, but instead form an integral part of the TPP development process.
The three key benefits of having a TPP are well described by the National Institutes of Health (NIH) SEED organization, as (1) ensuring enhanced R&D efficiency, (2) positive regulatory outcomes, and (3) commercial success [7]National Institutes of Health; SEED. Creating a Target Product Profile for New Drug Products. (accessed Sep 9, 2024).. It is the authors’ opinion that TPPs can also help qualify the intended value proposition and determine how the new CAR-T cell therapy will be differentiated from other therapies and standards of care by time of launch.
A TPP essentially serves as a roadmap for CAR-T cell developers, guiding decisions on clinical trial design, evidence generation strategies, and manufacturing [8]Wang T, McAuslane N, Goettsch WG, Leufkens HGM, De Bruin ML. Challenges and opportunities for companies to build HTA/payer perspectives into drug development through the use of a dynamic target product profile. Front. Pharmacol. 2022; 18(13), 948161.Wang T, McAuslane N, Goettsch WG, Leufkens HGM, De Bruin ML. Challenges and opportunities for companies to build HTA/payer perspectives into drug development through the use of a dynamic target product profile. Front. Pharmacol. 2022; 18(13), 948161.. The TPP may also inform go/no-go decisions at critical milestones as well as guide discussions between developers and regulatory and HTA authorities throughout the drug development process, from pre-IND phases to post-marketing programs and new indications or other substantial changes in regulatory labeling [5]US Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry and Review Staff Target Product Profile—A Strategic Development Process Tool. 2007. (accessed Sep 5, 2024).. As HTA now plays a key role in reimbursement decisions at a local level, and it will become clearer in due course how the single, coordinated EU-wide HTA process, known as Joint Clinical Assessment [9]Hague C. Navigating the EU joint clinical assessment process: key considerations for manufacturers of ATMPs and oncology medicines. Cell & Gene Therapy Insights 2024; 10(5), 717–728. will likely affect access to CAR-T cell and other oncology medicines, the TPP can also be used to inform the development of integrated evidence generation plans to meet HTA and payer requirements [8]Wang T, McAuslane N, Goettsch WG, Leufkens HGM, De Bruin ML. Challenges and opportunities for companies to build HTA/payer perspectives into drug development through the use of a dynamic target product profile. Front. Pharmacol. 2022; 18(13), 948161.Wang T, McAuslane N, Goettsch WG, Leufkens HGM, De Bruin ML. Challenges and opportunities for companies to build HTA/payer perspectives into drug development through the use of a dynamic target product profile. Front. Pharmacol. 2022; 18(13), 948161..
It has also been suggested to the authors that TPPs may be used by health authorities/payers as demand signaling documents on the types of innovations that are needed. This is also a very important benefit of having a TPP that can be used in external interactions between developers and key stakeholders.
How do I go about developing a TPP?
The WHO [10]World Health Organization. Target Product Profiles (TPPs) and Product Profile Characteristics (PPCs). 2022. (accessed Sep 7, 2024)., EMA [11]European Medicines Agency. ICH Guidance Q8 (R2) on Pharmaceutical Development (EMEA/CHMP/167068/2004). Jun 22, 2017. (accessed Sep 11, 2024)., and US FDA [5]US Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry and Review Staff Target Product Profile—A Strategic Development Process Tool. 2007. (accessed Sep 5, 2024). have all issued guidance documents on TPP development. The WHO describes a TPP development process that could be applied to a CAR-T therapy, with some minor amends suggested by the authors in bold italics. It recommends undertaking a needs assessment, appointing an internal cross-functional multi-disciplinary TPP team to draft an initial TPP, eliciting input (into the TPP) from patient expert organizations, clinicians, regulators and HTA agencies through the scientific advice processes, revising and finalizing the TPP with sign-off from an internal governance committee [12]Murtagh M, Blondeel K, Peeling RW, Kiarie J, Toskin I. The relevance of target product profiles for manufacturers, experiences from the World Health Organization initiative for point-of-care testing for sexually transmitted infections. Arch. Public Health 2021; 79(1), 187.. It is the authors’ opinion that the cross-functional team assigned to develop a TPP should consist of representatives from the following disciplines (e.g., project leadership, translational science, pharmacovigilance, product manufacturing, clinical development, regulatory affairs, health economics and outcomes research, epidemiology, real-world evidence, market access, pricing, medical affairs, marketing, and business insights). Note that different functions may be referred to differently within and across organizations and this list should be interpreted as purely illustrative of the types of functions that might be usefully represented. It is not meant to be a prescriptive nor complete list but provided to negate a perception that a TPP is a purely commercial or clinical deliverable. A TPP should be considered as an internally aligned, externally validated and cross-functionally developed deliverable.
This article proposes a template that better suits the unique nuances of CAR-T therapy, building on previous work by Becker et al. [13]Becker E, Bourgogne A, Neelapu SS. Planning a Phase 1 clinical trial: target product profile for a novel target CAR-T cell therapy. Open Works at MD Anderson 2021. (accessed Sep 9, 2024).Becker E, Bourgogne A, Neelapu SS. Planning a Phase 1 clinical trial: target product profile for a novel target CAR-T cell therapy. Open Works at MD Anderson 2021. (accessed Sep 9, 2024).. The template outlines categories for further treatment attributes beyond those described by the health authorities (Table 1). This template was based on previous work proposed by Becker et al. [13]Becker E, Bourgogne A, Neelapu SS. Planning a Phase 1 clinical trial: target product profile for a novel target CAR-T cell therapy. Open Works at MD Anderson 2021. (accessed Sep 9, 2024).Becker E, Bourgogne A, Neelapu SS. Planning a Phase 1 clinical trial: target product profile for a novel target CAR-T cell therapy. Open Works at MD Anderson 2021. (accessed Sep 9, 2024). NIH SEED [7]National Institutes of Health; SEED. Creating a Target Product Profile for New Drug Products. (accessed Sep 9, 2024). [14]National Institutes of Health (NIH); SEED. Example Target Product Profile (TPP) for a Cell and Gene Therapy. (accessed Sep 8, 2024). , and Hettle et al. [15]Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017; 21 (7), 1–204. and further adapted to include more specific information on the unmet need, the value proposition, the patient selection strategy including sub-groups, clinical efficacy, PROs, duration of the treatment-free interval, the pricing model, health economics, and patient access. These additional components were informed by the authors’ previous experience of developing TPPs for CAR-T cell therapies and the benefits they accrued through having thought through these aspects at an early stage of product development by having them explicitly set out within the TPP.
Table 1. Components of the clinical and production sections of the novel target CAR-T cell TPP. | |||
Sections | Components | Target product profile | Data sources/references |
Unmet need† | Statement of unmet need | << >> | << >> |
Value proposition† | Value proposition of the new CAR-T cell therapy | << >> | << >> |
Clinical | Proposed indication(s) | << >> | << >> |
Patient selection strategy (target population including sub-groups†) | << >> | << >> | |
Target safety and tolerability profile | << >> | << >> | |
Target clinical efficacy profile† | << >> | << >> | |
PROs† e.g., benefits in terms of health-related quality of life and symptom palliation | << >> | << >> | |
Optimal duration of the treatment-free interval† | << >> | << >> | |
Product characteristics | Stability and shelf life | << >> | << >> |
Route of administration | << >> | << >> | |
Dose and conditioning | << >> | << >> | |
Dosing frequency | << >> | << >> | |
Production | Critical quality attributes | << >> | << >> |
Cell viability and vector titer | << >> | << >> | |
Manufacturability | << >> | << >> | |
Apheresis logistics | << >> | << >> | |
Shipping and storage | << >> | << >> | |
CAR-T cell development | << >> | << >> | |
Lentivirus production | << >> | << >> | |
Turnaround time | << >> | << >> | |
Cost of goods | COGs | << >> | << >> |
Pricing model† | Target price (USA, EU5, rest of world)† | << >> | << >> |
Health economics† | Cost–effectiveness† | << >> | << >> |
Budget impact† | << >> | << >> | |
Patient access† | Managed entry agreements (MEAs), including price discounts, performance-related schemes, and technology leasing† | << >> | << >> |
Data from Becker et al. [13], NIH SEED [7,14] and Hettle et al. [15] and †further adapted. | |||
What does a good TPP look like?
Developing a TPP that serves to align developers’ and stakeholders’ perspectives will be highly valued if designed in the right way, such that it is able to minimize risk, reduce failure rates, anticipate the time needed to generate the necessary evidence and ideally reduce the costs of development [16]Boccardi M, Gold M, Mahant V, Marincola FM, Gunn A. Why should academia care about the Target Product Profile? J. Transl. Med. 2024; 22(1), 716. . Starting with the end-user in mind is fundamentally important [17]Lambert WJ. Considerations in developing a target product profile for parenteral pharmaceutical products. AAPS PharmSciTech 2010; 11(3), 1476–1481. . It is the authors’ opinion that early engagement with patient experts can enhance the quality of a TPP through better understanding those attributes of treatment most valued by the end user. This in turn can inform what should be captured in clinical trials to better understand the extent to which a new treatment might alleviate the burden of disease and improve patients’ health-related quality of life. Additionally, there are other CAR-T cell specific nuances where early patient expert engagement may be especially valuable, such as eliciting their views on the acceptability of manufacturing turnaround times, site locations for running trials and eventually for commercial use, proposed strategies for adverse event monitoring, and patient education materials amongst others. Stegemann et al. propose a roadmap to do this, which is highly recommended [18]Stegemann S, Sheehan L, Rossi A, et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—a proposed roadmap. Eur. J Pharm. Biopharm. 2022; 177, 81–88. .
Figure 2. 80 mm fig |
|---|
The figure is 80 mm |
Hettle et al. developed two TPPs in their exemplar work on CAR-T cell therapy in hematology which differentiated between ‘curative’ and ‘bridging to stem-cell transplantation’ treatment as the primary goal of treatment [15]Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017; 21 (7), 1–204. . This approach may also be very helpful to consider.
As far as specifying the attributes of treatment and the magnitude by which a CAR-T cell therapy has been able to demonstrate its superiority (i.e., the necessary ‘hurdle’ within the TPP that needs to be cleared), there are different schools of thought on this matter. Some suggest that a TPP should contain a minimally acceptable (essential) hurdle that specifies the minimal parameters for a safe and efficacious drug as well as a higher (ideal) hurdle that specifies the desirable parameters that would allow higher value (access to a larger market, reduced cost of goods, etc.) and hence defines a better commercially viable (optimal) benchmark [19]BioCurate. Constructing a Target Product Profile—Industry’s Perspective Fact Sheet. (accessed Sep 5, 2024)..
We maintain that the latter should be adopted as the ‘only’ TPP benchmark that developers should focus on, since many developers struggle to determine an appropriate course of action when a product falls short of meeting its optimal benchmark. A minimally acceptable hurdle may, at best, be equivalent to existing therapies, which makes decisions as to what to do next with a CAR-T in development much more difficult as payers tend to assign a much higher value to therapies that can offer patients improvements on standards of care, as opposed to ‘me-too’ (undifferentiated) treatments. Mobilizing efforts towards delivering to the optimal benchmark simplifies the assumptions underpinning the commercial forecast (e.g., adoption levels, market share, and price) and sets a necessary level of ambition within drug development teams for success [19]BioCurate. Constructing a Target Product Profile—Industry’s Perspective Fact Sheet. (accessed Sep 5, 2024).. Importantly, one should be aware of the possibility of trade-offs. This means to say that a different configuration of product attributes from that laid out in a TPP may generate a different value proposition and commercial forecast. Should a CAR-T cell therapy fall short of its TPP in one area, it should be clear whether this is something that can be offset by exceeding another domain within the TPP.
What is the optimal timing for developing a TPP?
Whilst the TPP is considered a ‘critical path’ tool by the FDA [5]US Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry and Review Staff Target Product Profile—A Strategic Development Process Tool. 2007. (accessed Sep 5, 2024)., research has found that TPPs are often developed too late in the process, usually at the time of the pre-non-disclosure agreement (NDA) or Biologics License Application (BLA) meeting or following NDA or BLA submission [20]Tyndall A, Du W, Breder CD. Regulatory watch: the target product profile as a tool for regulatory communication: advantageous but underused. Nat. Rev. Drug Discov. 2017; 16(3), 156. Tyndall A, Du W, Breder CD. Regulatory watch: the target product profile as a tool for regulatory communication: advantageous but underused. Nat. Rev. Drug Discov. 2017; 16(3), 156. . Companies finding themselves in this position miss out on valuable opportunities to streamline their interactions, which may result in a lengthier and more costly development process [20]Tyndall A, Du W, Breder CD. Regulatory watch: the target product profile as a tool for regulatory communication: advantageous but underused. Nat. Rev. Drug Discov. 2017; 16(3), 156. Tyndall A, Du W, Breder CD. Regulatory watch: the target product profile as a tool for regulatory communication: advantageous but underused. Nat. Rev. Drug Discov. 2017; 16(3), 156. .
It is the authors’ opinion that a TPP should ideally be in place by the time of read-out from the Phase 1 study for a CAR-T cell therapy. This is to ensure that the right health outcome data are collected during Phase 2 trials and beyond, and that any competitive and other insights can be factored into the trial design [8]Wang T, McAuslane N, Goettsch WG, Leufkens HGM, De Bruin ML. Challenges and opportunities for companies to build HTA/payer perspectives into drug development through the use of a dynamic target product profile. Front. Pharmacol. 2022; 18(13), 948161.. By preparing a TPP in this timely way, a more productive and fruitful dialogue can take place with health authorities using the TPP to guide discussions around the optimal design of clinical development and/or manufacturing plans for approval purposes.
How often should a TPP be updated?
A TPP is crucial in the fast-paced development of CAR-T cell therapies. It is the authors’ opinion that it should incorporate the latest evidence and competitive insights, including clinical efficacy, safety, and PROs. It should also include relevant insights from regulatory agencies, patient experts, and payers. The TPP should be considered a ‘live’ document, updated with new insights and information as they arise, whether they come from competitor data presented at conferences or termination of clinical trials for safety reasons.
What is an appropriate governance process for a TPP?
Once the TPP is drafted, it is important that developers have in place a formal approval mechanism as well as a change control system for any ongoing revisions to the template and to individual TPPs [21]Brooks A, Nunes JK, Garnette A, et al. Aligning new interventions with developing country health systems: target product profiles, presentation, and clinical trial design. Glob. Public Health 2012; 7(9), 931–945. . This preserves the integrity of the TPP and ensures that internal decisions whether to progress a CAR-T cell therapy into more advanced stages of clinical development are justified based on the evidence (i.e., what is being seen in clinical trials) and free from any perverse incentives (financial or otherwise) [18]Stegemann S, Sheehan L, Rossi A, et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—a proposed roadmap. Eur. J Pharm. Biopharm. 2022; 177, 81–88. Stegemann S, Sheehan L, Rossi A, et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—a proposed roadmap. Eur. J Pharm. Biopharm. 2022; 177, 81–88. . Without a governance process in place, a company may unwisely invest in a CAR-T cell therapy that will not achieve their commercial objectives [18]Stegemann S, Sheehan L, Rossi A, et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—a proposed roadmap. Eur. J Pharm. Biopharm. 2022; 177, 81–88. Stegemann S, Sheehan L, Rossi A, et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—a proposed roadmap. Eur. J Pharm. Biopharm. 2022; 177, 81–88. and where the opportunity cost is lost.
Which data and insights are needed to develop a robust TPP for a CAR-T cell therapy indicated for cancer?
Evidence-based insights should inform a TPP through undertaking a robust analysis of the published literature and emerging data on existing therapies as well as scrutiny of emerging competitor therapies under development.
The methodological steps that may be considered are as follows:
- Develop a protocol that sets out the inclusion/exclusion criteria that guide which studies ought to be included when formulating an appropriate benchmark within the TPP. The protocol might include certain restrictions on publication dates (to eliminate older studies), the phase of clinical development where later studies are prioritized over earlier phase studies), the line of treatment (where earlier lines are excluded if they do not reflect the target indication), and sample size (perhaps excluding studies with very small sample sizes).
- Identification of studies that meet the inclusion criteria set out in the study protocol that will likely result in a focused number of regulatory approved treatments and those treatment under development that are reporting promising data for the indication(s) targeted by the TPP from Pubmed/Medline.
- An analysis of treatment guidelines for the indication(s) identifying the current recommended treatment options for patients to ensure that no treatments have been missed from the list identified in step 2.
- Extraction of key efficacy, safety, and PRO data on those treatments into an Excel spreadsheet making it clear which phase of clinical development these values relate to, as well as the date of publication. This is important because later stage trials (e.g., Phase 3 and large Phase 2 trials) will often report more mature estimates from a larger sample size.
- Synthesis of the above data (median and IQR values) can help determine the benchmark within the TPP, against which the CAR-T cell under development can be meaningfully compared at different development milestones.
- An analysis of reimbursement outcomes for regulatory approved CAR-T cell and other treatments. This will likely uncover important insights on the hurdles other developers face when seeking reimbursement, so that these can be better characterized and mitigated against. For CAR-T cell therapies, these might include the uncertainties arising from non-comparative (single-arm) studies, the immaturity of time-to-event outcomes, trial exclusion criteria that limit the generalizability of data to a broader patient population in clinical practice, amongst others.
Patient experts may offer useful insights around the choice of PRO questionnaires for use in the clinical development program that they consider will best assess the side effects of treatment from a patient perspective and how the efficacy gains translate into an improvement in patients’ disease-related symptoms and quality of life. Eliciting insights from clinicians may also prove useful to contextualize the insights obtained from the methodological approach described above and provide guidance to the development of the protocol.
In addition to efficacy, safety, and PRO data extracted on those current standards against which the new CAR-T cell therapy will likely be compared by regulators and HTA agencies detailed descriptive information on patient characteristics may also be valuable, to help inform comparisons of the relative benefit/risk profile of the TPP versus alternative treatments.
Detailed analyses of ongoing and completed competitor clinical trials also help to identify areas where developers can differentiate themselves and at the same time, take advantage of the opportunity to align the design of their clinical studies to others to reduce bias when performing indirect treatment comparisons at a later stage. It is important to bear in mind that clinical practice evolves rapidly, and so developers should be mindful of incorporating older studies into their TPP benchmarks as these may reflect worse patient outcomes that might be typically observed by time of launch.
What are the key uncertainties and ‘watch outs’ when developing a TPP for a CAR-T cell therapy?
Like most innovative oncology therapies, there are a lot of uncertainties associated with early data, especially when studying orphan or ultra-orphan populations in a non-comparative manner.
Likelihood of cure
The likelihood of cure or improvement with the CAR-T cell therapy is a key uncertainty because it can take years to demonstrate as a result of having to wait for data maturity and can only likely be determined if no subsequent anti-cancer treatment is administered. For this reason, it is probably reasonable not to allude to an intent to cure within the TPP, but it is very important to be able to identify which patients respond best to treatment and understand the reasons why this might be the case.
Durability of treatment effect
Whilst use of CAR-T cell therapies has historically achieved very high overall response rates the onus is on the developer to demonstrate through adequate follow-up that the impressive responses are maintained over time, such that they translate into significant progression-free as well as overall survival, ideally with an extensive treatment-free window for patients. Trials with surrogate endpoints for overall survival tend to report larger treatment effects than trials using final patient-relevant outcomes, which obviously have implications when it comes to powering later-stage trials and ensuring adequate numbers of patients needed to treat [15]Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017; 21 (7), 1–204. . The pattern and duration of response to treatment can be uncertain if there is limited follow-up data available and so it is important that patients are followed for as long as feasibly possible [22]Hague C, Price M. Challenges and proposed solutions to value assessment and reimbursement of CAR-T therapies in Europe. Cell & Gene Therapy Insights 2020; 6(7), 1013–1028. .
Number of infusions and the effect of subsequent treatment on overall survival
The ‘one shot and done’ ethos of CAR-T cell therapy may not be sufficient for some patients to maintain their initial response and so it is important that developers consider how the economic value proposition of their product stacks up in the event that more than one infusion is needed [23]Golden D. Will a second infusion of CAR-T cells help against cancer? Alliance for Cancer Gene Therapy 2022. cell-therapy-infusion/#:~:text=One%20of%20the%20benefits%20of,that%20can%20persist%20for%20years (accessed Sep 5, 2024).. The effect of subsequent (non-cellular) therapy may also confound patients’ overall survival data, meaning that it is important that developers factor capturing these data into their clinical development plans.
Turnaround times
Manufacturing times should be a key consideration in TPP development to optimize patient outcomes, minimizing the turnaround time as much as possible. Given the potential deleterious impact of patients having to wait too long for their treatment, this should be a key consideration.
Magnitude of treatment effect versus current treatment options
Most CAR-T cell approvals have been granted based on non-comparative data. Consequently, the absence of randomized controlled trial (RCT) versus a standard of care makes it difficult to accurately determine what would likely be the magnitude of difference between arms in the event of an RCT. This is because estimates of relative efficacy sought through indirect comparisons (without any linking arms) can be subject to bias and potential confounding [22]Hague C, Price M. Challenges and proposed solutions to value assessment and reimbursement of CAR-T therapies in Europe. Cell & Gene Therapy Insights 2020; 6(7), 1013–1028. . In this situation, developers need to consider how best to generate these comparative data and which standards of care should be studied.
Size of the commercial opportunity
The TPP can also help to create clarity around the likely size of the target population for the new treatment by describing the proposed indication and where within the treatment pathway it will likely be positioned. Epidemiological data needs to be adjusted to reflect the line of therapy, the requirement of specified prior treatments, and study inclusion criteria (e.g., performance status, age cut-offs, stage of disease, etc.) that may exclude certain types of patients. Figure 2 illustrates the starting point for such a calculation using the example of multiple myeloma. These data would need to be further adjusted to reflect the likely market share of the new treatment over time, given the clinical attractiveness of the TPP, how the TPP stacks up versus established and emerging standards of care and the target price.
FIGUre 2 |
Global incidence and prevalence of multiple myeloma. |
Adapted from [31]. |
Deciding on what determines an appropriate standard of care
Depending on the disease in question, there may be considerable heterogeneity as to what constitutes the standard of care, with wide variation in both the costs and consequences of multiple different treatment options (as was observed in the real-world LocoMMotion study of patients with multiple myeloma who had been exposed to three classes of treatment that was undertaken to support the development of ciltacabtagene autoleucel [cilta-cel]) [24]Mateos MV, Weisel K, Martin T, et al. Adjusted comparison of outcomes between patients from CARTITUDE-1 versus multiple myeloma patients with prior exposure toproteasome inhibitors, immunomodulatory drugs and anti-CD38 antibody from the prospective, multinational LocoMMotion study of real-world clinical practice. Haematologica 2023; 108(8), 2192–2204. . This means to say that it is important to isolate what constitutes the dominant standard of care in key sources of business markets guided by robust analyses of the latest treatment guidelines (Figure 3).
FIGUre 3 |
Multiple myeloma treatment algorithm derived from the National Comprehensive Cancer Network (NCCN) guidelines [32]. |
Immaturity of data for decision-making
A key challenge when developing a TPP for a CAR-T cell therapy is figuring out how emerging data on a CAR-T in early development is likely to stack up versus a competitive benchmark that reflects a more mature estimate of efficacy and safety. It is important to be able to map out the trajectory of data maturity for competitor CAR-Ts and standards of care at their respective stages of clinical development such that meaningful comparisons can be performed for go/no-go decisions. The issue of data maturity serves as an important reminder that not everything can be specified within a TPP. There will likely be both known and unknown unknowns. The key question therefore is what to do when data immaturity is a known unknown? This is to be differentiated from missing data, but clarified as key time to event data from patients who are still benefiting from treatment such that they remain alive, and ideally free of progressive disease.
Landmark analyses can be extremely useful in this scenario [25]Dafni U. Landmark analysis at the 25-year landmark point. Circ. Cardiovasc. Qual. Outcomes 2011; 4(3), 363–371. . Immaturity of data also poses challenges for HTA agencies that use cost-utility analyses to help inform their decision making since quality-adjusted survival is highly uncertain where both the overall survival data AND utility data are subject to extrapolation (modeling) assumptions, that have a knock-on effect on the robustness of any resulting cost-effectiveness estimates of a CAR-T cell therapy versus standard of care in patients with different health states [26]Jonsson B, Hampson G, Michaels J, et al. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur. J. Health Econ. 2019; 20(3), 427–438. [27]Lin JK, Muffly LS, Spinner M, et al. Cost effectiveness of chimeric antigen receptor T cell therapy in multiple relapsed or refractory adult large b-cell lymphoma. J. Clin. Oncol. 2019; 37(24), 2105–2119. [28]Raymakers AJN, Regier DA, Peacock SJ. Modelling uncertainty in survival and cost–effectiveness is vital in the era of gene therapies: the case of axicabtagene ciloleucel. Health Policy Technol. 2019; 8(2): 104–104. [29]Gibson EJ, Begum N, Koblbauer I, et al. Modeling the economic outcomes of immuno-oncology drugs: alternative model frameworks to capture clinical outcomes. ClinicoEcon. Outcomes Res. 2018; 10, 139–154..
Hettle et al. suggests a way to accommodate data maturity through defining three data sets [15]Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017; 21 (7), 1–204. ; however, it is important to note that one needs to adjust the TPP benchmarks accordingly for each set for it to be meaningful for decision-making purposes.
- The minimum set: the minimum data considered potentially sufficient for CAR-T cell therapy to be granted conditional regulatory approval.
- The intermediate set: a variant of the minimum set in which the efficacy and safety of CAR-T cell therapy have been assessed over a longer follow-up period.
- The mature set: a variant of the intermediate set in which the efficacy and safety of CAR-T cell therapy have been assessed in a larger clinical study but with a similar follow-up period as in the intermediate set.
Model estimates of long-term outcomes, in the absence of observation, are likely to be highly uncertain. For this reason, developers may need to draw on different sources of evidence to inform their modeling assumptions and perhaps consider extending the follow-up period in their clinical trials to be in a strong position to be able to validate their modeling assumptions at a later point in time.
Reliability of comparative evidence in the absence of randomized controlled trial data
Obtaining robust historical ‘control arm’ data that is a perfect match with the cohort of patients included in the single arm trial can be challenging [15]Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017; 21 (7), 1–204. . Developers are advised to invest carefully in studies that attempt to generate comparable data on standards of care that measure the same endpoints (as captured in their trial), using the same definitions and captured at the same time, as well as ensuring that the patient characteristics are as similar as feasibly possible in terms of their stage of disease, number of prior therapies, performance status and any other important prognostic factors.
Assigning weights to TPP attributes
Not taking the opportunity to externally validate a TPP is a frequent omission of many companies. Furthermore, failing to consider the relative weight of their TPP attributes to commercial success. It is important that companies gain a quantitative understanding of what TPP attributes are the primary drivers of differentiated value that will translate to preferential market shares (and those that are ‘nice to have’). The TPP attributes will not likely be valued equally and so ranking them in order of importance could be useful, especially when it comes to making investment decisions at the various stages of development. Different methods can be explored for weighting purposes (e.g., using qualitative means such as Delphi techniques) but the more robust the approach to weighting and valuing individual TPP attributes, the more informed the decision-making process becomes). Ultimately, the pursuit of developing commercially unviable treatments carries with it a significant opportunity cost which should be avoided wherever possible.
Limitations of the paper
The authors set out to describe what they consider to be critical success factors for commercially viable therapies and acknowledge that the way they have approached this is from determining what success looks like at launch and working backwards. Success is defined as CAR-T cell therapies that reach those patients that could benefit most from treatment by clearing the market access and payer hurdles through demonstrating a superior offering to alternative treatments where the value-based price can be justified accordingly. The authors accept that some manufacturers may prefer to restrict the focus of their TPP on clearing the first hurdle, namely regulatory approval as it makes for a less complex undertaking. However, incorporating important market access considerations into a TPP at an early stage enables manufacturers to better understand the higher hurdles that may be involved in securing reimbursement, and hence a return on investment. It also helps to better inform a more reliable and accurate commercial forecast based on a TPP that is optimized in this way.
A further limitation of the paper is that the authors do not allude to the challenges associated with the adoption of CAR-T cell therapies, such as how they might fit within clinical care pathways in different countries, the infrastructure that is needed within hospitals to accommodate these types of treatment, together with workforce capacity constraints and any needed capability (skills) development. These are clearly very important considerations for developers of CAR-T cell therapies.
Translation insights
In summary, there are many uncertainties when developing a CAR-T cell therapy and a TPP aligning developers and key stakeholders can help to minimize risk, failure rates, time needed, and costs. Ideally, a TPP should be in place by the time of initiating the Phase 1 study for internal decision-making purposes, as well as helping to inform discussions with regulatory authorities, HTA agencies, payers, and patients. A formal approval mechanism and change control systems are advised to preserve the integrity of a TPP and ensure that go-no/go decisions can be justified based on the evidence, using the TPP benchmark as a yardstick for enhanced R&D efficiency, positive regulatory outcomes, and commercial success.
References
1. Yu JX, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019; 18(12), 899–900. Crossref
2. National Institutes for Health. Clinicaltrials.gov search results. (accessed Sep 5, 2024). Link
3. Markets and Markets. CAR-T cell therapy market size, share and trends. cell-therapy-market-47772841.html#:~:text=The%20size%20of%20global%20CAR,39.6%25%20from%202024%20to%202029 (accessed Sep 9, 2024). Link
4. Stanton D. Cost of goods is crucial for the future of regenerative medicine: CAR-T cell therapy provides a case study in perspective. BioProcess International Apr 18, 2019. (accessed Sep 9, 2024). Link
5. US Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry and Review Staff Target Product Profile—A Strategic Development Process Tool. 2007. (accessed Sep 5, 2024). Link
6. US Food and Drug Administration; Center for Drug Evaluation Research; Center for Biologics Evaluation Research. Guidance for Industry. Q8(R2) Pharmaceutical Development. Nov 2009. (accessed Sep 5, 2024). Link
7. National Institutes of Health; SEED. Creating a Target Product Profile for New Drug Products. (accessed Sep 9, 2024). Link
8. Wang T, McAuslane N, Goettsch WG, Leufkens HGM, De Bruin ML. Challenges and opportunities for companies to build HTA/payer perspectives into drug development through the use of a dynamic target product profile. Front. Pharmacol. 2022; 18(13), 948161. Crossref
9. Hague C. Navigating the EU joint clinical assessment process: key considerations for manufacturers of ATMPs and oncology medicines. Cell & Gene Therapy Insights 2024; 10(5), 717–728. Crossref
10. World Health Organization. Target Product Profiles (TPPs) and Product Profile Characteristics (PPCs). 2022. (accessed Sep 7, 2024). Link
11. European Medicines Agency. ICH Guidance Q8 (R2) on Pharmaceutical Development (EMEA/CHMP/167068/2004). Jun 22, 2017. (accessed Sep 11, 2024). Link
12. Murtagh M, Blondeel K, Peeling RW, Kiarie J, Toskin I. The relevance of target product profiles for manufacturers, experiences from the World Health Organization initiative for point-of-care testing for sexually transmitted infections. Arch. Public Health 2021; 79(1), 187. Crossref
13. Becker E, Bourgogne A, Neelapu SS. Planning a Phase 1 clinical trial: target product profile for a novel target CAR-T cell therapy. Open Works at MD Anderson 2021. (accessed Sep 9, 2024). Link
14. National Institutes of Health (NIH); SEED. Example Target Product Profile (TPP) for a Cell and Gene Therapy. (accessed Sep 8, 2024). Link
15. Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017; 21 (7), 1–204. Crossref
16. Boccardi M, Gold M, Mahant V, Marincola FM, Gunn A. Why should academia care about the Target Product Profile? J. Transl. Med. 2024; 22(1), 716. Crossref
17. Lambert WJ. Considerations in developing a target product profile for parenteral pharmaceutical products. AAPS PharmSciTech 2010; 11(3), 1476–1481. Crossref
18. Stegemann S, Sheehan L, Rossi A, et al. Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes—a proposed roadmap. Eur. J Pharm. Biopharm. 2022; 177, 81–88. Crossref
19. BioCurate. Constructing a Target Product Profile—Industry’s Perspective Fact Sheet. (accessed Sep 5, 2024). Link
20. Tyndall A, Du W, Breder CD. Regulatory watch: the target product profile as a tool for regulatory communication: advantageous but underused. Nat. Rev. Drug Discov. 2017; 16(3), 156. Crossref
21. Brooks A, Nunes JK, Garnette A, et al. Aligning new interventions with developing country health systems: target product profiles, presentation, and clinical trial design. Glob. Public Health 2012; 7(9), 931–945. Crossref
22. Hague C, Price M. Challenges and proposed solutions to value assessment and reimbursement of CAR-T therapies in Europe. Cell & Gene Therapy Insights 2020; 6(7), 1013–1028. Crossref
23. Golden D. Will a second infusion of CAR-T cells help against cancer? Alliance for Cancer Gene Therapy 2022. cell-therapy-infusion/#:~:text=One%20of%20the%20benefits%20of,that%20can%20persist%20for%20years (accessed Sep 5, 2024). Link
24. Mateos MV, Weisel K, Martin T, et al. Adjusted comparison of outcomes between patients from CARTITUDE-1 versus multiple myeloma patients with prior exposure toproteasome inhibitors, immunomodulatory drugs and anti-CD38 antibody from the prospective, multinational LocoMMotion study of real-world clinical practice. Haematologica 2023; 108(8), 2192–2204. Crossref
25. Dafni U. Landmark analysis at the 25-year landmark point. Circ. Cardiovasc. Qual. Outcomes 2011; 4(3), 363–371. Crossref
26. Jonsson B, Hampson G, Michaels J, et al. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur. J. Health Econ. 2019; 20(3), 427–438. Crossref
27. Lin JK, Muffly LS, Spinner M, et al. Cost effectiveness of chimeric antigen receptor T cell therapy in multiple relapsed or refractory adult large b-cell lymphoma. J. Clin. Oncol. 2019; 37(24), 2105–2119. Crossref
28. Raymakers AJN, Regier DA, Peacock SJ. Modelling uncertainty in survival and cost–effectiveness is vital in the era of gene therapies: the case of axicabtagene ciloleucel. Health Policy Technol. 2019; 8(2): 104–104. Crossref
29. Gibson EJ, Begum N, Koblbauer I, et al. Modeling the economic outcomes of immuno-oncology drugs: alternative model frameworks to capture clinical outcomes. ClinicoEcon. Outcomes Res. 2018; 10, 139–154. Crossref
30. GlobalData. Trials Intelligence Database (accessed Sep 19, 2024). Crossref
31. GlobalData. Multiple Myeloma Epidemiology (report code: GDHCER314) 2023. (accessed Sep 12, 2024). Crossref
32. Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 2 2024. J. Natl. Compr. Canc. Netw. 2023; 21(12), 1281–1301. Crossref
Affiliations
Clare Hague PhD
Managing Director,
Oncology Access Solutions Ltd,
Cheshire, UK
(Author for correspondence)
chague@oncologyaccesssolutions.co.uk
Louise Street-Docherty PhD
Vice President Strategic Intelligence,
VISFO,
Lincolnshire, UK
Frances Pearson DPhil
Senior Director Strategic Intelligence,
VISFO,
Lincolnshire, UK
Authorship & Conflict of Interest
Contributions: The named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements:The authors would like to thank GlobalData for their support in providing their permission for the use of data sets extracted and analyzed from the subscription service data platform to derive the figures for this article. We also wish to thank the peer reviewers of this article for their valuable feedback.
Disclosure and potential conflicts of interest: Clare Hague is a Trustee of Radiotherapy UK and is a holder of stock options in Johnson & Johnson.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright:Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2024 Oncology Access Solutions Ltd. Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Oct 3, 2024; Revised manuscript received: Dec 2, 2024; Publication date: Dec 19, 2024.