
Welcome to Cell and Gene Therapy Insights’s Learning Center, brought to you in conjunction with Thermo Fisher Scientific. This extensive library of our cell and gene therapy content is designed to help you find the support you need to overcome obstacles, streamline processes, and advance your cell and gene therapies.
Content

Implementing a closed cell therapy manufacturing process through strategic collaboration
Øystein Åmellem, Xavier de Mollerat du Jeu, Brian Shy
13 April 2023
Innovator Insight

Enhancing non-viral gene editing, processing & expansion of T & NK cells
6 February 2023
Innovator Insight

Regulatory requirements and product attributes for cGMP viral vector production
Scott Cross, Shikha Mishra
19 December 2022
Poster

Enhancing non-viral genome editing, processing, and expansion of T and NK cells
Sung Lee, Deepak Kumar
8 December 2022
Watch
Regulatory requirements and product attributes for cGMP viral vector production
Scott Cross, Shikha Mishra
20 October 2022
Watch

Feeder free expansion and closed wash and concentration of a clinically relevant number of human NK cells
Erica Heipertz PhD
29 September 2022
Watch

A closed, modular approach to autologous CAR T cell therapy manufacturing
Jason Isaacson
13 September 2022
FastFacts
Automated and scalable closed-system platform for cell isolation and activation
Hany Meås
31 August 2022
Watch
Scaling non-viral cell therapy approaches for solid tumor treatments
Evan Zynda, PhD, Nektaria Andronikou
31 August 2022
Poster

Build vs buy dilemma: economics of manufacturing cell-based therapies
Nirupama (Rupa) Pike
28 July 2022
Watch
Scalable solutions for cell isolation and expansion
Evan Zynda
18 July 2022
Poster
The digital revolution: technological innovations to enable automation in cell therapy manufacturing
Bruce Greenwald, Sean Chang, Krish Roy
13 July 2022
Poster

Applying an HPLC analytical platform for mRNA process monitoring
Nejc Pavlin
7 June 2022
Watch
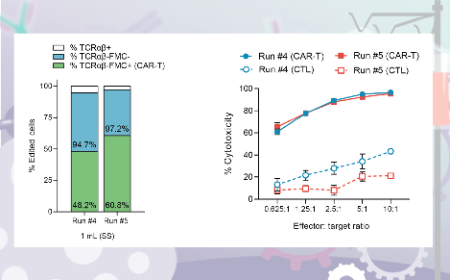
Improving T cell therapy manufacturing processes with automation and scalability
Evan Zynda PhD
26 May 2022
Watch
Scaling non-viral cell therapy approaches for solid tumor treatments
Evan Zynda, Nektaria Andronikou, Kyle Jacoby
23 May 2022
Innovator Insight

Strategic partnering to enable cell therapy commercialization
Jenessa Smith, Xavier de Mollerat du Jeu
12 May 2022
Watch
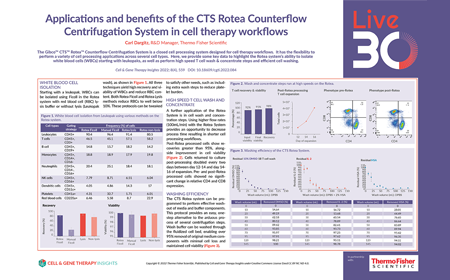
Applications and benefits of the CTS Rotea Counterflow Centrifugation System in cell therapy workflows
Carl Dargitz
11 May 2022
Poster
Supply chain efficiency and sourcing for scaling your gene therapy operation
Céline Martin, Don Young
3 May 2022
Poster

Applications and benefits of the CTS Rotea Counterflow Centrifugation System in cell therapy workflows
Carl Dargitz
13 April 2022
Watch
The digital revolution: technological innovations to enable automation in cell therapy manufacturing
Sean Chang, Bruce Greenwald, Krishnendu Roy
12 April 2022
Innovator Insight

Supply chain efficiency and sourcing for scaling your gene therapy operation
Celine Martin, Don Young
7 April 2022
Watch

Scaling non-viral cell therapy approaches for solid tumor treatments
N Andronikou, E Zynda PhD, K Jacoby PhD
24 March 2022
Watch
The digital revolution: Technological innovations to enable automation in cell therapy manufacturing
S Chang, B Greenwald, K Roy
3 February 2022
Watch

Achieving cost-effective, scalable high-titer AAV production
Chao Yan Liu
30 September 2021
FastFacts

Applying a closed, modular, semi-automated system to CAR T cell therapy manufacturing
Yongchang Ji
14 September 2021
Innovator Insight

Applying a closed, modular, semi-automated system to CAR T cell therapy manufacturing
Y Ji PhD, S Ensari PhD, N Moore PhD
29 June 2021
Watch

Exploring the capabilities of a versatile, novel, automated closed system for cell and gene therapy manufacturing
Sarah Daoudi, Premkumar Jayaraman
25 March 2021
Innovator Insight
Exploring the capabilities of a versatile, novel, automated closed system for cell and gene therapy manufacturing
Premkumar Jayaraman PhD, Sarah Daoudi
16 February 2021
Watch