Process and analytical insights for GMP manufacturing of mRNA lipid nanoparticles

The successful development and rapid deployment of the messenger RNA (mRNA) vaccines against SARS-CoV-2 virus has catalyzed the industry to look more closely at the technology beyond its potential use for novel vaccines to enable breakthrough treatments for cancer, rare diseases, and more. However, the absence of standardized protocols means manufacturers must develop and optimize their process, leading to a considerable number of variables and decisions throughout the production workflow.
This webinar will discuss key insights into the potential of the mRNA and lipid nanoparticle (LNP) technologies that underpin the COVID-19 vaccines, barriers to successful industrialized manufacture of mRNA–LNPs, and approaches to optimizing process development and manufacturing. The panel will address:
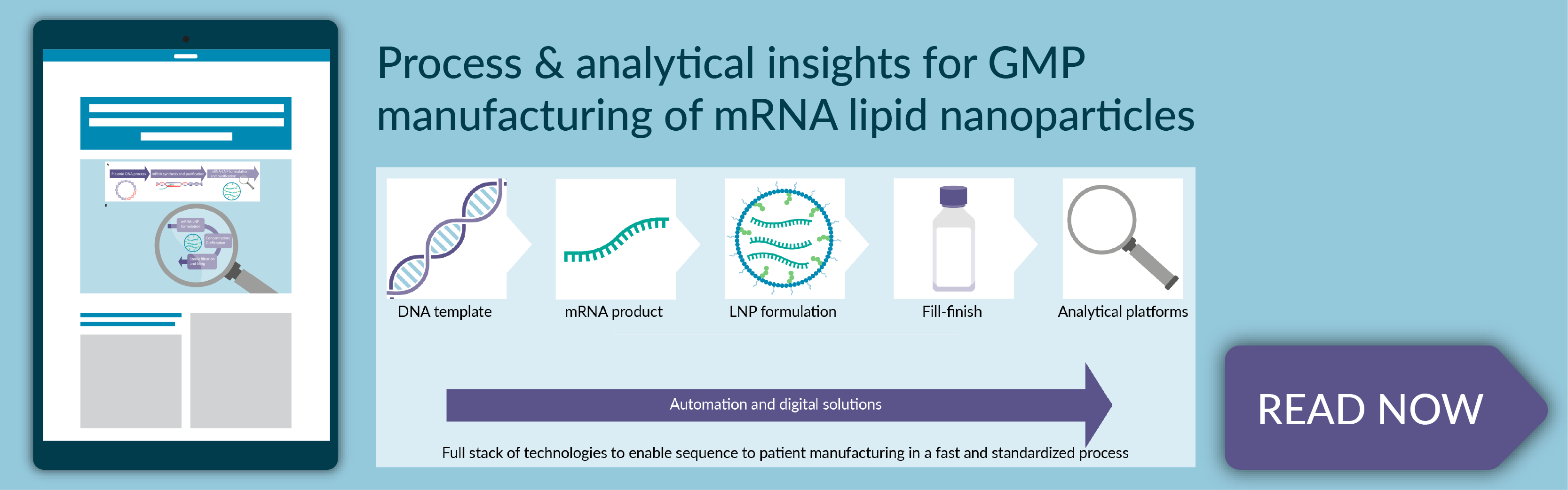
- The 5 steps of manufacturing mRNA-LNP drug products, including DNA template manufacturing, mRNA drug substance synthesis and purification, mRNA–LNP formulation and purification, fill/finish operations, and analytical testing
- Process and analytical considerations for GMP manufacturing of mRNA-LNPs
- Challenges and opportunities for mRNA-LNP therapeutic development
You might also like

Achieving cost effective, scalable high-titer AAV production
Hit the lentiviral characterization jackpot with accurate RNA detection

Mastering allogeneic NK cell therapies: flexibility, robustness, and speed to clinic

Biological & operational considerations for iPSC-derived cell production






